Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium
- PMID: 9531551
- PMCID: PMC2132716
- DOI: 10.1083/jcb.141.1.101
Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium
Abstract
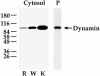
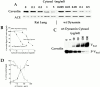
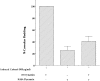
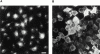
The molecular mechanisms mediating cell surface trafficking of caveolae are unknown. Caveolae bud from plasma membranes to form free carrier vesicles through a "pinching off" or fission process requiring cytosol and driven by GTP hydrolysis (Schnitzer, J.E., P. Oh, and D.P. McIntosh. 1996. Science. 274:239-242). Here, we use several independent techniques and functional assays ranging from cell-free to intact cell systems to establish a function for dynamin in the formation of transport vesicles from the endothelial cell plasma membrane by mediating fission at the neck of caveolae. This caveolar fission requires interaction with cytosolic dynamin as well as its hydrolysis of GTP. Expression of dynamin in cytosol as well as purified recombinant dynamin alone supports GTP-induced caveolar fission in a cell-free assay whereas its removal from cytosol or the addition to the cytosol of specific antibodies for dynamin inhibits this fission. Overexpression of mutant dynamin lacking normal GTPase activity not only inhibits GTP-induced fission and budding of caveolae but also prevents caveolae-mediated internalization of cholera toxin B chain in intact and permeabilized endothelial cells. Analysis of endothelium in vivo by subcellular fractionation and immunomicroscopy shows that dynamin is concentrated on caveolae, primarily at the expected site of action, their necks. Thus, through its ability to oligomerize, dynamin appears to form a structural collar around the neck of caveolae that hydrolyzes GTP to mediate internalization via the fission of caveolae from the plasma membrane to form free transport vesicles.
Figures
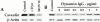

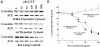
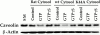


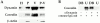




Similar articles
-
Role of GTP hydrolysis in fission of caveolae directly from plasma membranes.Science. 1996 Oct 11;274(5285):239-42. doi: 10.1126/science.274.5285.239. Science. 1996. PMID: 8824187
-
Expression of mutant dynamin protects cells against diphtheria toxin but not against ricin.Exp Cell Res. 1998 Mar 15;239(2):293-300. doi: 10.1006/excr.1997.3921. Exp Cell Res. 1998. PMID: 9521846
-
Dynamin mediates caveolar sequestration of muscarinic cholinergic receptors and alteration in NO signaling.EMBO J. 2000 Aug 15;19(16):4272-80. doi: 10.1093/emboj/19.16.4272. EMBO J. 2000. PMID: 10944110 Free PMC article.
-
Dynamin GTPase, a force-generating molecular switch.Bioessays. 1996 Nov;18(11):885-93. doi: 10.1002/bies.950181107. Bioessays. 1996. PMID: 8939066 Review.
-
Dynamin and its role in membrane fission.Annu Rev Cell Dev Biol. 2000;16:483-519. doi: 10.1146/annurev.cellbio.16.1.483. Annu Rev Cell Dev Biol. 2000. PMID: 11031245 Free PMC article. Review.
Cited by
-
Endocytic pathways involved in filovirus entry: advances, implications and future directions.Viruses. 2012 Dec;4(12):3647-64. doi: 10.3390/v4123647. Viruses. 2012. PMID: 23342373 Free PMC article. Review.
-
Taking the Scenic Route: Polyomaviruses Utilize Multiple Pathways to Reach the Same Destination.Viruses. 2020 Oct 15;12(10):1168. doi: 10.3390/v12101168. Viruses. 2020. PMID: 33076363 Free PMC article. Review.
-
Targeting caveolae to pump bispecific antibody to TGF-β into diseased lungs enables ultra-low dose therapeutic efficacy.PLoS One. 2022 Nov 22;17(11):e0276462. doi: 10.1371/journal.pone.0276462. eCollection 2022. PLoS One. 2022. PMID: 36413536 Free PMC article.
-
Acid sensitive background potassium channels K2P3.1 and K2P9.1 undergo rapid dynamin-dependent endocytosis.Channels (Austin). 2013 Jul-Aug;7(4):288-302. doi: 10.4161/chan.25120. Epub 2013 Jun 10. Channels (Austin). 2013. PMID: 23807092 Free PMC article.
-
EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization.Mol Biol Cell. 2012 Apr;23(7):1316-29. doi: 10.1091/mbc.E11-09-0787. Epub 2012 Feb 9. Mol Biol Cell. 2012. PMID: 22323287 Free PMC article.
References
-
- Chen MS, Obar RA, Schroeder CC, Austin TW, Poodry CA, Wadsworth SC, Vallee RB. Multiple forms of dynamin are encoded by shibire, a Drosophilia gene involved in endocytosis. Nature. 1991;351:583–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

