Chronic inflammation and susceptibility to bacterial infections in mice lacking the polypeptide (p)105 precursor (NF-kappaB1) but expressing p50
- PMID: 9529315
- PMCID: PMC2212206
- DOI: 10.1084/jem.187.7.985
Chronic inflammation and susceptibility to bacterial infections in mice lacking the polypeptide (p)105 precursor (NF-kappaB1) but expressing p50
Abstract
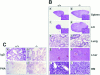
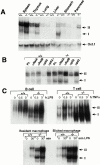
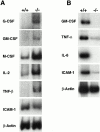
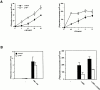
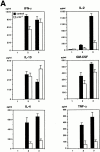
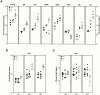
The polypeptide (p)50 molecule, a subunit of nuclear factor (NF)-kappaB, is produced after proteolytic processing of the p105 precursor (NF-kappaB1). Although the p105 precursor has been postulated to play a role in the regulation of the Rel/NF-kappaB activity, its physiological relevance remains unclear. To investigate that, we generated mutant mice lacking the COOH terminal half of the p105 precursor, but expressing the p50 product (p105-/-). These mutant mice displayed an inflammatory phenotype composed of lymphocytic infiltration in lungs and liver, and an increased susceptibility to opportunistic infections. Enlargement of multiple lymph nodes, splenomegaly due to erythrocytic extramedullary hematopoiesis, and lymphoid hyperplasia were also observed in p105-/- mice. Cytokine production in p105-/- macrophages was severely impaired, whereas proliferative responses of p105-/- B cells were increased. T cell functions were only moderately impaired in mutant mice. Loss of p105 also led to enhanced constitutive p50 homodimer and inducible NF-kappaB activities in unstimulated and stimulated cells, respectively. As several genes regulated by Rel/NF-kappaB were upregulated in p105-/- thymus but downregulated in p105-/- macrophages, the enhanced p50 homodimers appear to function as transcriptional activators or repressors, depending on the cell type. Thus, the p105 precursor is indispensable in the control of p50 activity, and lack of the precursor has distinct effects on different cells.
Figures





Similar articles
-
Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology.Biochem J. 2004 Sep 1;382(Pt 2):393-409. doi: 10.1042/BJ20040544. Biochem J. 2004. PMID: 15214841 Free PMC article. Review.
-
Characterization of ES cells deficient for the p105 precursor (NF-kappa B1): role of p50 NLS.Oncogene. 1996 Jul 18;13(2):255-63. Oncogene. 1996. PMID: 8710364
-
TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105.Nature. 1999 Jan 28;397(6717):363-8. doi: 10.1038/16946. Nature. 1999. PMID: 9950430
-
Promoter of the human NF-kappa B p50/p105 gene. Regulation by NF-kappa B subunits and by c-REL.J Immunol. 1993 Apr 1;150(7):2794-804. J Immunol. 1993. PMID: 8454856
-
NFKB1: a suppressor of inflammation, ageing and cancer.FEBS J. 2016 May;283(10):1812-22. doi: 10.1111/febs.13627. Epub 2016 Jan 13. FEBS J. 2016. PMID: 26663363 Review.
Cited by
-
Absence of IκBβ/NFκB signaling does not attenuate acetaminophen-induced hepatic injury.Anat Rec (Hoboken). 2022 Nov 25:10.1002/ar.25126. doi: 10.1002/ar.25126. Online ahead of print. Anat Rec (Hoboken). 2022. PMID: 36426684 Free PMC article.
-
RelB is required for Peyer's patch development: differential regulation of p52-RelB by lymphotoxin and TNF.EMBO J. 2003 Jan 2;22(1):121-30. doi: 10.1093/emboj/cdg004. EMBO J. 2003. PMID: 12505990 Free PMC article.
-
Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology.Biochem J. 2004 Sep 1;382(Pt 2):393-409. doi: 10.1042/BJ20040544. Biochem J. 2004. PMID: 15214841 Free PMC article. Review.
-
Cotranslational dimerization of the Rel homology domain of NF-kappaB1 generates p50-p105 heterodimers and is required for effective p50 production.EMBO J. 2000 Sep 1;19(17):4712-22. doi: 10.1093/emboj/19.17.4712. EMBO J. 2000. PMID: 10970863 Free PMC article.
-
The NF-kappaB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes.J Hepatol. 2010 Sep;53(3):519-27. doi: 10.1016/j.jhep.2010.03.025. Epub 2010 Jun 2. J Hepatol. 2010. PMID: 20579762 Free PMC article.
References
-
- Grilli M, Chiu J-S, Lenardo MJ. NF-κB and Rel-participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–62. - PubMed
-
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
-
- Kopp EB, Ghosh S. NF-κB and Rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. - PubMed
-
- Miyamoto S, Verma IM. Rel/NF–κB/IκB story. Adv Cancer Res. 1995;66:255–292. - PubMed
-
- Beg AA, Baldwin AS., Jr The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes Dev. 1993;7:2063–2070. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

