Chlamydial infection in inducible nitric oxide synthase knockout mice
- PMID: 9529043
- PMCID: PMC108050
- DOI: 10.1128/IAI.66.4.1282-1286.1998
Chlamydial infection in inducible nitric oxide synthase knockout mice
Abstract
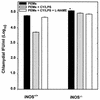
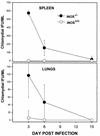
Type 1 CD4+-T-cell-mediated immunity is crucial for the resolution of chlamydial infection of the murine female genital tract. Previous studies demonstrating a correlation between CD4+-T-cell-mediated inhibition of chlamydial growth and gamma interferon (IFN-gamma)-mediated induction of nitric oxide synthase suggested a potential role for the nitric oxide (NO) effector pathway in the clearance of Chlamydia from genital epithelial cells by the immune system. To clarify the role of this pathway, the growth levels of Chlamydia trachomatis organisms in normal (iNOS+/+) mice and in genetically engineered mice lacking the inducible nitric oxide synthase (iNOS) gene (iNOS-/- mice) were compared. There was no significant difference in the course of genital chlamydial infections in iNOS+/+ and iNOS-/- mice as determined by recovery of Chlamydia organisms shed from genital epithelial cells. Dissemination of Chlamydia to the spleen and lungs occurred to a greater extent in iNOS-/- than in iNOS+/+ mice, which correlated with a marginal increase in the susceptibility of macrophages from iNOS-/- mice to chlamydial infection in vitro. However, infections were rapidly cleared from all affected tissues, with no clinical signs of disease. The finding of minimal dissemination in iNOS-/- mice suggested that activation of the iNOS effector pathway was not the primary target of IFN-gamma during CD4+-T-cell-mediated control of chlamydial growth in macrophages because previous reports demonstrated extensive and often fatal dissemination of Chlamydia in mice lacking IFN-gamma. In summary, these results indicate that the iNOS effector pathway is not required for elimination of Chlamydia from epithelial cells lining the female genital tract of mice although it may contribute to the control of dissemination of C. trachomatis by infected macrophages.
Figures
Similar articles
-
Inducible nitric oxide synthase does not affect resolution of murine chlamydial genital tract infections or eradication of chlamydiae in primary murine cell culture.Infect Immun. 1998 Feb;66(2):835-8. doi: 10.1128/IAI.66.2.835-838.1998. Infect Immun. 1998. PMID: 9453651 Free PMC article.
-
Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome.Infect Immun. 2001 Aug;69(8):5131-7. doi: 10.1128/IAI.69.8.5131-5137.2001. Infect Immun. 2001. PMID: 11447195 Free PMC article.
-
Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response.Infect Immun. 1997 Mar;65(3):1032-44. doi: 10.1128/IAI.65.3.1032-1044.1997. Infect Immun. 1997. PMID: 9038313 Free PMC article.
-
The role of IFN-gamma in the outcome of chlamydial infection.Curr Opin Immunol. 2002 Aug;14(4):444-51. doi: 10.1016/s0952-7915(02)00361-8. Curr Opin Immunol. 2002. PMID: 12088678 Review.
-
Nitric oxide synthases and tubal ectopic pregnancies induced by Chlamydia infection: basic and clinical insights.Mol Hum Reprod. 2010 Dec;16(12):907-15. doi: 10.1093/molehr/gaq063. Epub 2010 Jul 20. Mol Hum Reprod. 2010. PMID: 20647263 Free PMC article. Review.
Cited by
-
Role of catalase in Campylobacter jejuni intracellular survival.Infect Immun. 2000 Nov;68(11):6337-45. doi: 10.1128/IAI.68.11.6337-6345.2000. Infect Immun. 2000. PMID: 11035743 Free PMC article.
-
The quantity of nitric oxide released by macrophages regulates Chlamydia-induced disease.Proc Natl Acad Sci U S A. 2002 Mar 19;99(6):3914-9. doi: 10.1073/pnas.062578399. Proc Natl Acad Sci U S A. 2002. PMID: 11904441 Free PMC article.
-
Genetic Screen in Chlamydia muridarum Reveals Role for an Interferon-Induced Host Cell Death Program in Antimicrobial Inclusion Rupture.mBio. 2019 Apr 9;10(2):e00385-19. doi: 10.1128/mBio.00385-19. mBio. 2019. PMID: 30967464 Free PMC article.
-
Macrophage Polarization during Murine Lyme Borreliosis.Infect Immun. 2015 Jul;83(7):2627-35. doi: 10.1128/IAI.00369-15. Epub 2015 Apr 13. Infect Immun. 2015. PMID: 25870230 Free PMC article.
-
Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection.Infect Immun. 2008 Feb;76(2):515-22. doi: 10.1128/IAI.01064-07. Epub 2007 Nov 19. Infect Immun. 2008. PMID: 18025098 Free PMC article.
References
-
- Adams L B, Hibbs J B, Taintor R R, Krahenbuhl J L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii: role of synthesis of inorganic nitrogen oxides from l-arginine. J Immunol. 1990;144:2725–2729. - PubMed
-
- Boehm U, Klamp T, Groot M, Howard J C. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

