Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus
- PMID: 9525617
- PMCID: PMC109742
- DOI: 10.1128/JVI.72.4.2962-2968.1998
Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus
Abstract
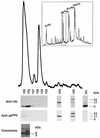
Host proteins are incorporated into retroviral virions during assembly and budding. We have examined three retroviruses, human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus (SIV), and Moloney murine leukemia virus (Mo-MuLV), for the presence of ubiquitin inside each of these virions. After a protease treatment to remove exterior viral as well as contaminating cellular proteins, the proteins remaining inside the virion were analyzed. The results presented here show that all three virions incorporate ubiquitin molecules at approximately 10% of the level of Gag found in virions. In addition to free ubiquitin, covalent ubiquitin-Gag complexes were detected, isolated, and characterized from all three viruses. Our immunoblot and protein sequencing results on treated virions showed that approximately 2% of either HIV-1 or SIV p6Gag was covalently attached to a single ubiquitin molecule inside the respective virions and that approximately 2 to 5% of the p12Gag in Mo-MuLV virions was monoubiquitinated. These results show that ubiquitination of Gag is conserved among these retroviruses and occurs in the p6Gag portion of the Gag polyprotein, a region that is likely to be involved in assembly and budding.
Figures
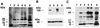
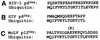

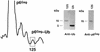
Similar articles
-
Ubiquitination of HIV-1 and MuLV Gag.Virology. 2000 Dec 5;278(1):111-21. doi: 10.1006/viro.2000.0648. Virology. 2000. PMID: 11112487
-
The virion-associated Gag-Pol is decreased in chimeric Moloney murine leukemia viruses in which the readthrough region is replaced by the frameshift region of the human immunodeficiency virus type 1.Virology. 2005 Apr 10;334(2):342-52. doi: 10.1016/j.virol.2005.01.044. Virology. 2005. PMID: 15780884
-
Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus.J Virol. 2002 Jun;76(11):5315-25. doi: 10.1128/jvi.76.11.5315-5325.2002. J Virol. 2002. PMID: 11991960 Free PMC article.
-
Incorporation of human immunodeficiency virus type 1 Gag proteins into murine leukemia virus virions.J Virol. 1993 Nov;67(11):6499-506. doi: 10.1128/JVI.67.11.6499-6506.1993. J Virol. 1993. PMID: 8411353 Free PMC article.
-
Specific incorporation of cyclophilin A into HIV-1 virions.Nature. 1994 Nov 24;372(6504):359-62. doi: 10.1038/372359a0. Nature. 1994. PMID: 7969494
Cited by
-
The HIV-1 gag p6: a promising target for therapeutic intervention.Retrovirology. 2024 Jan 23;21(1):1. doi: 10.1186/s12977-024-00633-2. Retrovirology. 2024. PMID: 38263239 Free PMC article. Review.
-
Biochemical and proteomic characterization of retrovirus Gag based microparticles carrying melanoma antigens.Sci Rep. 2016 Jul 11;6:29425. doi: 10.1038/srep29425. Sci Rep. 2016. PMID: 27403717 Free PMC article.
-
How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain.Viruses. 2020 Aug 13;12(8):888. doi: 10.3390/v12080888. Viruses. 2020. PMID: 32823718 Free PMC article. Review.
-
The nucleoside triphosphate diphosphohydrolase-1/CD39 is incorporated into human immunodeficiency type 1 particles, where it remains biologically active.J Mol Biol. 2007 Aug 3;371(1):269-82. doi: 10.1016/j.jmb.2007.05.012. Epub 2007 May 10. J Mol Biol. 2007. PMID: 17560607 Free PMC article.
-
Mutational analysis of the C-terminal gag cleavage sites in human immunodeficiency virus type 1.J Virol. 2007 Sep;81(18):10047-54. doi: 10.1128/JVI.02496-06. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634233 Free PMC article.
References
-
- Arthur L O, Bess J W, Jr, Sowder II R C, Benveniste R E, Mann L D, Chermann J-C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. - PubMed
-
- Ball E, Karlik C C, Beall C J, Saville D L, Sparrow J C, Bullard B, Fyrberg E A. Arthrin, a myofibrillar protein of insect flight muscle, is an actin-ubiquitin conjugate. Cell. 1987;51:221–228. - PubMed
-
- Bess J W, Jr, Gorelick R J, Bosche W J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. - PubMed
-
- Chau V, Tobias J W, Bachmair A, Marriott D, Ecker D J, Gonda D K, Varshavsky A. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Nature. 1989;243:1576–1583. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

