Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia
- PMID: 9501225
- PMCID: PMC19704
- DOI: 10.1073/pnas.95.6.3117
Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia
Abstract
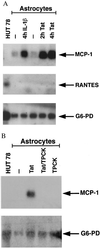
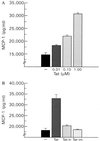
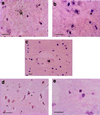
Activated monocytes release a number of substances, including inflammatory cytokines and eicosanoids, that are highly toxic to cells of the central nervous system. Because monocytic infiltration of the central nervous system closely correlates with HIV-1-associated dementia, it has been suggested that monocyte-derived toxins mediate nervous system damage. In the present study, we show that the HIV-1 transactivator protein Tat significantly increases astrocytic expression and release of monocyte chemoattractant protein-1 (MCP-1). Astrocytic release of beta-chemokines, which are relatively less selective for monocytes, including RANTES, macrophage inflammatory protein-1alpha, and macrophage inflammatory protein-1beta, was not observed. We also show that MCP-1 is expressed in the brains of patients with HIV-1-associated dementia and that, of the beta-chemokines tested, only MCP-1 could be detected in the cerebrospinal fluid of patients with this condition. Together, these data provide a potential link between the presence of HIV-1 in the brain and the monocytic infiltration that may substantially contribute to dementia.
Figures


Similar articles
-
MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis.J Neurochem. 2003 Jun;85(5):1299-311. doi: 10.1046/j.1471-4159.2003.01775.x. J Neurochem. 2003. PMID: 12753088
-
Expression of chemokines by human fetal microglia after treatment with the human immunodeficiency virus type 1 protein Tat.J Neurovirol. 2004 Apr;10(2):86-97. doi: 10.1080/13550280490279807. J Neurovirol. 2004. PMID: 15204927
-
HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes.J Immunol. 1999 Sep 1;163(5):2953-9. J Immunol. 1999. PMID: 10453044
-
MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression.J Leukoc Biol. 1997 Jul;62(1):30-3. doi: 10.1002/jlb.62.1.30. J Leukoc Biol. 1997. PMID: 9225989 Review.
-
HIV tat and neurotoxicity.Microbes Infect. 2006 Apr;8(5):1347-57. doi: 10.1016/j.micinf.2005.11.014. Epub 2006 Jan 26. Microbes Infect. 2006. PMID: 16697675 Review.
Cited by
-
Protease resistant protein cellular isoform (PrP(c)) as a biomarker: clues into the pathogenesis of HAND.J Neuroimmune Pharmacol. 2013 Dec;8(5):1159-66. doi: 10.1007/s11481-013-9458-4. Epub 2013 Apr 25. J Neuroimmune Pharmacol. 2013. PMID: 23616272 Free PMC article. Review.
-
Altered expression of fractalkine in HIV-1-infected astrocytes and consequences for the virus-related neurotoxicity.J Neurovirol. 2021 Apr;27(2):279-301. doi: 10.1007/s13365-021-00955-3. Epub 2021 Mar 1. J Neurovirol. 2021. PMID: 33646495
-
Β-funaltrexamine inhibits chemokine (CXCL10) expression in normal human astrocytes.Neurochem Int. 2013 Mar;62(4):478-85. doi: 10.1016/j.neuint.2013.01.013. Epub 2013 Jan 31. Neurochem Int. 2013. PMID: 23376103 Free PMC article.
-
CD4-independent entry and replication of simian immunodeficiency virus in primary rhesus macaque astrocytes are regulated by the transmembrane protein.J Virol. 2005 Apr;79(8):4944-51. doi: 10.1128/JVI.79.8.4944-4951.2005. J Virol. 2005. PMID: 15795280 Free PMC article.
-
Association of platelet-derived growth factor-B chain with simian human immunodeficiency virus encephalitis.Am J Pathol. 2004 Sep;165(3):815-24. doi: 10.1016/S0002-9440(10)63344-5. Am J Pathol. 2004. PMID: 15331406 Free PMC article.
References
-
- Price R W, Brew B, Sidtis J, Rosenblum M, Scheck A C, Cleary P. Science. 1988;239:586–592. - PubMed
-
- Glass J D, Fedor H S, Wesselingh S L, McArthur J C. Ann Neurol. 1995;38:755–762. - PubMed
-
- Gartner S, Markovits P, Markovitz D, Kaplan M, Gallo R, Popovic M. Science. 1986;233:215–219. - PubMed
-
- Koenig S, Gendelman H E, Orenstein J M, DalCanto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Science. 1986;233:1089–1093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

