Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock
- PMID: 9500794
- PMCID: PMC2212185
- DOI: 10.1084/jem.187.6.917
Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock
Abstract
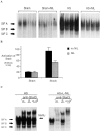
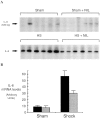
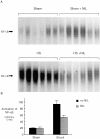
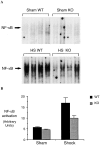
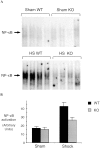
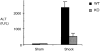
Resuscitation from hemorrhagic shock induces profound changes in the physiologic processes of many tissues and activates inflammatory cascades that include the activation of stress transcriptional factors and upregulation of cytokine synthesis. This process is accompanied by acute organ damage (e.g., lungs and liver). We have previously demonstrated that the inducible nitric oxide synthase (iNOS) is expressed during hemorrhagic shock. We postulated that nitric oxide production from iNOS would participate in proinflammatory signaling. Using the iNOS inhibitor N6-(iminoethyl)-L-lysine or iNOS knockout mice we found that the activation of the transcriptional factors nuclear factor kappaB and signal transducer and activator of transcription 3 and increases in IL-6 and G-CSF messenger RNA levels in the lungs and livers measured 4 h after resuscitation from hemorrhagic shock were iNOS dependent. Furthermore, iNOS inhibition resulted in a marked reduction of lung and liver injury produced by hemorrhagic shock. Thus, induced nitric oxide is essential for the upregulation of the inflammatory response in resuscitated hemorrhagic shock and participates in end organ damage under these conditions.
Figures









Similar articles
-
Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock.Am J Physiol Gastrointest Liver Physiol. 2004 Feb;286(2):G225-33. doi: 10.1152/ajpgi.00447.2002. Am J Physiol Gastrointest Liver Physiol. 2004. PMID: 14715517
-
Novel roles of nitric oxide in hemorrhagic shock.Shock. 1999 Jul;12(1):1-9. doi: 10.1097/00024382-199907000-00001. Shock. 1999. PMID: 10468045 Review.
-
Distinct effects of systemic infusion of G-CSF vs. IL-6 on lung and liver inflammation and injury in hemorrhagic shock.Shock. 2000 Jul;14(1):41-8. doi: 10.1097/00024382-200014010-00008. Shock. 2000. PMID: 10909892
-
A nitric oxide scavenger protects against pulmonary inflammation following hemorrhagic shock.Shock. 2002 Feb;17(2):98-103. doi: 10.1097/00024382-200202000-00003. Shock. 2002. PMID: 11837796
-
Molecular mechanisms in the early phase of hemorrhagic shock.Langenbecks Arch Surg. 2001 Jul;386(4):302-8. doi: 10.1007/s004230100242. Langenbecks Arch Surg. 2001. PMID: 11466573 Free PMC article. Review.
Cited by
-
Nitric oxide and redox regulation in the liver: part II. Redox biology in pathologic hepatocytes and implications for intervention.J Surg Res. 2011 May 1;167(1):96-112. doi: 10.1016/j.jss.2009.10.006. Epub 2009 Oct 27. J Surg Res. 2011. PMID: 20400112 Free PMC article. Review.
-
Activation of nuclear factor kappaB and induction of inducible nitric oxide synthase by Ureaplasma urealyticum in macrophages.Infect Immun. 2000 Dec;68(12):7087-93. doi: 10.1128/IAI.68.12.7087-7093.2000. Infect Immun. 2000. PMID: 11083834 Free PMC article.
-
Akt-mediated signaling is induced by cytokines and cyclic adenosine monophosphate and suppresses hepatocyte inducible nitric oxide synthase expression independent of MAPK P44/42.Biochim Biophys Acta. 2011 Jan;1813(1):73-9. doi: 10.1016/j.bbamcr.2010.10.001. Epub 2010 Oct 8. Biochim Biophys Acta. 2011. PMID: 20934465 Free PMC article.
-
Acute alcohol intoxication reduces mortality, inflammatory responses and hepatic injury after haemorrhage and resuscitation in vivo.Br J Pharmacol. 2012 Feb;165(4b):1188-99. doi: 10.1111/j.1476-5381.2011.01595.x. Br J Pharmacol. 2012. PMID: 21790532 Free PMC article.
-
Trauma hemorrhagic shock-induced lung injury involves a gut-lymph-induced TLR4 pathway in mice.PLoS One. 2011;6(8):e14829. doi: 10.1371/journal.pone.0014829. Epub 2011 Aug 4. PLoS One. 2011. PMID: 21829592 Free PMC article.
References
-
- Chaudry, I.H., W. Ertel, and A. Ayala. 1993. Alterations in inflammatory cytokine production following hemorrhage and resuscitation. In Shock, Sepsis, and Organ Failure, Third Wiggers Bernard Conference. G. Schlag, H. Redl, and D.L. Traber, editors. Springer Verlag, Berlin. 73–127.
-
- Cotran, R.S., V. Kumar, and S.L. Robbins, editors. 1989. Robbins Pathologic Basis of Disease. 4th ed. W.B. Saunders Company, Philadelphia. 39–71.
-
- Deitch EA, Morrison J, Berg R, Specian RD. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med. 1990;18:529–536. - PubMed
-
- Peitzman AB, Udekwu AO, Ochoa J, Smith S. Bacterial translocation in trauma patients. J Trauma. 1991;31:1083–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

