Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity
- PMID: 9499111
- PMCID: PMC109550
- DOI: 10.1128/JVI.72.3.2491-2495.1998
Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity
Abstract
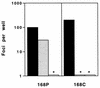
We have examined the relationship between coreceptor utilization and sensitivity to neutralization in a primary isolate of human immunodeficiency virus type 1 and its T-cell line-adapted (TCLA) derivative. We determined that adaptation of the primary-isolate (PI) virus 168P results in the loss of the unique capacity of PI viruses to utilize the CCR5 coreceptor and in the acquisition by the TCLA 168C virus of sensitivity to neutralization by V3-directed monoclonal antibodies (MAbs). In experiments wherein infection by 168P is directed via either the CCR5 or the CXCR4 pathway, we demonstrate that the virus, as well as pseudotyped virions bearing a molecularly cloned 168P envelope protein, remains refractory to neutralization by MAbs 257-D, 268-D, and 50.1 regardless of the coreceptor utilized. This study suggests that coreceptor utilization is not a primary determinant of differential neutralization sensitivity in PI and TCLA viruses.
Figures
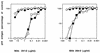


Similar articles
-
Continued utilization of CCR5 coreceptor by a newly derived T-cell line-adapted isolate of human immunodeficiency virus type 1.J Virol. 1998 Sep;72(9):7603-8. doi: 10.1128/JVI.72.9.7603-7608.1998. J Virol. 1998. PMID: 9696861 Free PMC article.
-
Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains.J Virol. 2005 Jun;79(11):6957-68. doi: 10.1128/JVI.79.11.6957-6968.2005. J Virol. 2005. PMID: 15890935 Free PMC article.
-
Differential CD4/CCR5 utilization, gp120 conformation, and neutralization sensitivity between envelopes from a microglia-adapted human immunodeficiency virus type 1 and its parental isolate.J Virol. 2001 Apr;75(8):3568-80. doi: 10.1128/JVI.75.8.3568-3580.2001. J Virol. 2001. PMID: 11264346 Free PMC article.
-
Protection of neutralization epitopes in the V3 loop of oligomeric human immunodeficiency virus type 1 glycoprotein 120 by N-linked oligosaccharides in the V1 region.AIDS Res Hum Retroviruses. 2001 Jul 20;17(11):1067-76. doi: 10.1089/088922201300343753. AIDS Res Hum Retroviruses. 2001. PMID: 11485624
-
Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1.J Virol. 1998 Dec;72(12):9855-64. doi: 10.1128/JVI.72.12.9855-9864.1998. J Virol. 1998. PMID: 9811721 Free PMC article.
Cited by
-
Increased neutralization sensitivity and reduced replicative capacity of human immunodeficiency virus type 1 after short-term in vivo or in vitro passage through chimpanzees.J Virol. 2000 Sep;74(17):7699-707. doi: 10.1128/jvi.74.17.7699-7707.2000. J Virol. 2000. PMID: 10933675 Free PMC article.
-
Genetic evidence that interhelical packing interactions in the gp41 core are critical for transition of the human immunodeficiency virus type 1 envelope glycoprotein to the fusion-active state.J Virol. 2002 Jul;76(14):7356-62. doi: 10.1128/jvi.76.14.7356-7362.2002. J Virol. 2002. PMID: 12072535 Free PMC article.
-
Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein.J Virol. 2000 Sep;74(17):7922-35. doi: 10.1128/jvi.74.17.7922-7935.2000. J Virol. 2000. PMID: 10933700 Free PMC article.
-
A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor.J Virol. 1999 Nov;73(11):8966-74. doi: 10.1128/JVI.73.11.8966-8974.1999. J Virol. 1999. PMID: 10516002 Free PMC article.
-
Characterization of primary isolate-like variants of simian-human immunodeficiency virus.J Virol. 1999 Dec;73(12):10199-207. doi: 10.1128/JVI.73.12.10199-10207.1999. J Virol. 1999. PMID: 10559336 Free PMC article.
References
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Berger, E. A. 1997. HIV entry and tropism: the chemokine receptor connection. AIDS 11(Suppl. A):S3–S16. - PubMed
-
- Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. - PubMed
-
- Broder C C, Dimitrov D S, Blumenthal R, Berger E A. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component. Virology. 1993;193:483–491. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

