Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis
- PMID: 9490736
- PMCID: PMC2132684
- DOI: 10.1083/jcb.140.5.1255
Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis
Abstract
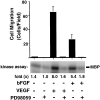
Angiogenesis depends on growth factors and vascular cell adhesion events. Integrins and growth factors are capable of activating the ras/MAP kinase pathway in vitro, yet how these signals influence endothelial cells during angiogenesis is unknown. Upon initiation of angiogenesis with basic fibroblast growth factor (bFGF) on the chick chorioallantoic membrane (CAM), endothelial cell mitogen-activated protein (MAP) kinase (ERK) activity was detected as early as 5 min yet was sustained for at least 20 h. The initial wave of ERK activity (5-120 min) was refractory to integrin antagonists, whereas the sustained activity (4-20 h) depended on integrin alphavbeta3, but not beta1 integrins. Inhibition of MAP kinase kinase (MEK) during this sustained alphavbeta3-dependent ERK signal blocked the formation of new blood vessels while not influencing preexisting blood vessels on the CAM. Inhibition of MEK also blocked growth factor induced migration but not adhesion of endothelial cells in vitro. Therefore, angiogenesis depends on sustained ERK activity regulated by the ligation state of both a growth factor receptor and integrin alphavbeta3.
Figures






Similar articles
-
Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis.J Cell Biol. 2003 Sep 1;162(5):933-43. doi: 10.1083/jcb.200304105. J Cell Biol. 2003. PMID: 12952943 Free PMC article.
-
Functional overlap and cooperativity among alphav and beta1 integrin subfamilies during skin angiogenesis.J Invest Dermatol. 2003 Jun;120(6):1100-9. doi: 10.1046/j.1523-1747.2003.12236.x. J Invest Dermatol. 2003. PMID: 12787141
-
Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner.J Cell Biol. 1999 Jul 12;146(1):149-64. doi: 10.1083/jcb.146.1.149. J Cell Biol. 1999. PMID: 10402467 Free PMC article.
-
Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis.J Clin Invest. 1996 Jul 15;98(2):426-33. doi: 10.1172/JCI118808. J Clin Invest. 1996. PMID: 8755653 Free PMC article.
-
Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin.Am J Pathol. 2000 Apr;156(4):1345-62. doi: 10.1016/s0002-9440(10)65005-5. Am J Pathol. 2000. PMID: 10751360 Free PMC article.
Cited by
-
The ERK mitogen-activated protein kinase pathway contributes to Ebola virus glycoprotein-induced cytotoxicity.J Virol. 2007 Feb;81(3):1230-40. doi: 10.1128/JVI.01586-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108034 Free PMC article.
-
Suppression of ras-mediated transformation and inhibition of tumor growth and angiogenesis by anthrax lethal factor, a proteolytic inhibitor of multiple MEK pathways.Proc Natl Acad Sci U S A. 2001 Mar 27;98(7):4089-94. doi: 10.1073/pnas.061031898. Epub 2001 Mar 20. Proc Natl Acad Sci U S A. 2001. PMID: 11259649 Free PMC article.
-
NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin.Mol Biol Cell. 2004 Aug;15(8):3580-90. doi: 10.1091/mbc.e04-03-0236. Epub 2004 Jun 4. Mol Biol Cell. 2004. PMID: 15181153 Free PMC article.
-
Inhibition of RAF dimers: it takes two to tango.Biochem Soc Trans. 2021 Feb 26;49(1):237-251. doi: 10.1042/BST20200485. Biochem Soc Trans. 2021. PMID: 33367512 Free PMC article. Review.
-
Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells.Mol Cell Biochem. 2013 Aug;380(1-2):1-9. doi: 10.1007/s11010-013-1651-5. Epub 2013 Apr 24. Mol Cell Biochem. 2013. PMID: 23613227
References
-
- Alessi D, Cuenda A, Cohen P, Dudley D, Saltiel A. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Boulton T, Nye S, Robbins D, Ip N, Radziejewska E, Morgenbesser S, DePinho R, Panayotatos N, Cobb M, Yancopoulos G. ERKs: a family of protein serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

