Hepatocyte nuclear factor 4 provokes expression of epithelial marker genes, acting as a morphogen in dedifferentiated hepatoma cells
- PMID: 9472044
- PMCID: PMC2141753
- DOI: 10.1083/jcb.140.4.935
Hepatocyte nuclear factor 4 provokes expression of epithelial marker genes, acting as a morphogen in dedifferentiated hepatoma cells
Abstract
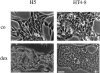
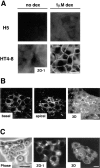
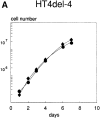
We have recently shown that stable expression of an epitope-tagged cDNA of the hepatocyte- enriched transcription factor, hepatocyte nuclear factor (HNF)4, in dedifferentiated rat hepatoma H5 cells is sufficient to provoke reexpression of a set of hepatocyte marker genes. Here, we demonstrate that the effects of HNF4 expression extend to the reestablishment of differentiated epithelial cell morphology and simple epithelial polarity. The acquisition of epithelial morphology occurs in two steps. First, expression of HNF4 results in reexpression of cytokeratin proteins and partial reestablishment of E-cadherin production. Only the transfectants are competent to respond to the synthetic glucocorticoid dexamethasone, which induces the second step of morphogenesis, including formation of the junctional complex and expression of a polarized cell phenotype. Cell fusion experiments revealed that the transfectant cells, which show only partial restoration of E-cadherin expression, produce an extinguisher that is capable of acting in trans to downregulate the E-cadherin gene of well-differentiated hepatoma cells. Bypass of this repression by stable expression of E-cadherin in H5 cells is sufficient to establish some epithelial cell characteristics, implying that the morphogenic potential of HNF4 in hepatic cells acts via activation of the E-cadherin gene. Thus, HNF4 seems to integrate the genetic programs of liver-specific gene expression and epithelial morphogenesis.
Figures





Similar articles
-
Hepatocyte nuclear factor 4 expression overcomes repression of the hepatic phenotype in dedifferentiated hepatoma cells.Mol Cell Biol. 1997 Apr;17(4):1913-22. doi: 10.1128/MCB.17.4.1913. Mol Cell Biol. 1997. PMID: 9121439 Free PMC article.
-
Phenotypic effects of the forced expression of HNF4 and HNF1alpha are conditioned by properties of the recipient cell.J Cell Sci. 1998 Aug;111 ( Pt 16):2411-21. doi: 10.1242/jcs.111.16.2411. J Cell Sci. 1998. PMID: 9683635
-
Liver-enriched transcription factors uncoupled from expression of hepatic functions in hepatoma cell lines.Mol Cell Biol. 1997 Nov;17(11):6311-20. doi: 10.1128/MCB.17.11.6311. Mol Cell Biol. 1997. PMID: 9343392 Free PMC article.
-
The role of E-cadherin and scatter factor in tumor invasion and cell motility.EXS. 1991;59:109-26. doi: 10.1007/978-3-0348-7494-6_8. EXS. 1991. PMID: 1833225 Review.
-
[Hepatocyte nuclear factor 4 (HNF4) in epithelial development and carcinogenesis].Mol Biol (Mosk). 2008 Sep-Oct;42(5):786-97. Mol Biol (Mosk). 2008. PMID: 18988528 Review. Russian.
Cited by
-
The nuclear receptor hepatocyte nuclear factor 4alpha acts as a morphogen to induce the formation of microvilli.J Cell Biol. 2006 Dec 18;175(6):971-80. doi: 10.1083/jcb.200608012. J Cell Biol. 2006. PMID: 17178913 Free PMC article.
-
Aberrant expression of alternative isoforms of transcription factors in hepatocellular carcinoma.World J Hepatol. 2018 Oct 27;10(10):645-661. doi: 10.4254/wjh.v10.i10.645. World J Hepatol. 2018. PMID: 30386458 Free PMC article. Review.
-
An HNF4α-microRNA-194/192 signaling axis maintains hepatic cell function.J Biol Chem. 2017 Jun 23;292(25):10574-10585. doi: 10.1074/jbc.M117.785592. Epub 2017 May 2. J Biol Chem. 2017. PMID: 28465351 Free PMC article.
-
Transcriptional regulation of cell adhesion at the blood-testis barrier and spermatogenesis in the testis.Adv Exp Med Biol. 2012;763:281-94. doi: 10.1007/978-1-4614-4711-5_14. Adv Exp Med Biol. 2012. PMID: 23397630 Free PMC article. Review.
-
Hepatocyte polarity.Compr Physiol. 2013 Jan;3(1):243-87. doi: 10.1002/cphy.c120009. Compr Physiol. 2013. PMID: 23720287 Free PMC article. Review.
References
-
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. - PubMed
-
- Amicone L, Spagnoli FM, Späth G, Giordano S, Tommasini C, Bernardini S, De Luca V, Della C, Rocca, Weiss MC, Comoglio PM, Tripodi M. Transgenic expression in the liver of truncated Met blocks apoptosis and permits immortalization of hepatocytes. EMBO (Eur Mol Biol Organ) J. 1997;16:495–503. - PMC - PubMed
-
- Angrand PO, Kallenbach S, Weiss MC, Rousset JP. An exogenous albumin promoter can become silent in dedifferentiated hepatoma variants as well as intertypic hybrids. Cell Growth Differ. 1990;1:519–526. - PubMed
-
- Baffet G, Loyer P, Glaise D, Corlu A, Etienne PL, Guguen GC. Distinct effects of cell-cell communication, and corticosteroids on the synthesis and distribution of cytokeratins in cultured rat hepatocytes. J Cell Sci. 1991;99:609–615. - PubMed
-
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

