A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate
- PMID: 9445060
- PMCID: PMC124638
- DOI: 10.1128/JVI.72.2.1577-1585.1998
A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate
Abstract
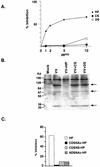
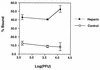
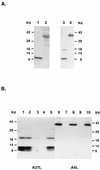
Vaccinia virus has a wide host range and infects mammalian cells of many different species. This suggests that the cell surface receptors for vaccinia virus are ubiquitously expressed and highly conserved. Alternatively, different receptors are used for vaccinia virus infection of different cell types. Here we report that vaccinia virus binds to heparan sulfate, a glycosaminoglycan (GAG) side chain of cell surface proteoglycans, during virus infection. Soluble heparin specifically inhibits vaccinia virus binding to cells, whereas other GAGs such as condroitin sulfate or dermantan sulfate have no effect. Heparin also blocks infections by cowpox virus, rabbitpox virus, myxoma virus, and Shope fibroma virus, suggesting that cell surface heparan sulfate could be a general mediator of the entry of poxviruses. The biochemical nature of the heparin-blocking effect was investigated. Heparin analogs that have acetyl groups instead of sulfate groups also abolish the inhibitory effect, suggesting that the negative charges on GAGs are important for virus infection. Furthermore, BSC40 cells treated with sodium chlorate to produce undersulfated GAGs are more refractory to vaccinia virus infection. Taken together, the data support the notion that cell surface heparan sulfate is important for vaccinia virus infection. Using heparin-Sepharose beads, we showed that vaccinia virus virions bind to heparin in vitro. In addition, we demonstrated that the recombinant A27L gene product binds to the heparin beads in vitro. This recombinant protein was further shown to bind to cells, and such interaction could be specifically inhibited by soluble heparin. All the data together indicated that A27L protein could be an attachment protein that mediates vaccinia virus binding to cell surface heparan sulfate during viral infection.
Figures


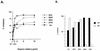

Similar articles
-
Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells.J Virol. 1999 Oct;73(10):8750-61. doi: 10.1128/JVI.73.10.8750-8761.1999. J Virol. 1999. PMID: 10482629 Free PMC article.
-
Cell surface proteoglycans are necessary for A27L protein-mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain.J Virol. 1998 Oct;72(10):8374-9. doi: 10.1128/JVI.72.10.8374-8379.1998. J Virol. 1998. PMID: 9733888 Free PMC article.
-
A Genome-Wide Haploid Genetic Screen Identifies Heparan Sulfate-Associated Genes and the Macropinocytosis Modulator TMED10 as Factors Supporting Vaccinia Virus Infection.J Virol. 2019 Jun 14;93(13):e02160-18. doi: 10.1128/JVI.02160-18. Print 2019 Jul 1. J Virol. 2019. PMID: 30996093 Free PMC article.
-
The oligomeric structure of vaccinia viral envelope protein A27L is essential for binding to heparin and heparan sulfates on cell surfaces: a structural and functional approach using site-specific mutagenesis.J Mol Biol. 2005 Jun 24;349(5):1060-71. doi: 10.1016/j.jmb.2005.04.024. J Mol Biol. 2005. PMID: 15913650
-
Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus.Adv Exp Med Biol. 1992;313:341-53. doi: 10.1007/978-1-4899-2444-5_33. Adv Exp Med Biol. 1992. PMID: 1332443 Review.
Cited by
-
Functional epitopes and neutralizing antibodies of vaccinia virus.Front Microbiol. 2023 Oct 25;14:1255935. doi: 10.3389/fmicb.2023.1255935. eCollection 2023. Front Microbiol. 2023. PMID: 37954238 Free PMC article. Review.
-
Integrin β1 mediates vaccinia virus entry through activation of PI3K/Akt signaling.J Virol. 2012 Jun;86(12):6677-87. doi: 10.1128/JVI.06860-11. Epub 2012 Apr 11. J Virol. 2012. PMID: 22496232 Free PMC article.
-
Structural and functional analyses of viral H2 protein of the vaccinia virus entry fusion complex.J Virol. 2023 Dec 21;97(12):e0134323. doi: 10.1128/jvi.01343-23. Epub 2023 Nov 17. J Virol. 2023. PMID: 37975688 Free PMC article.
-
Smallpox vaccines: targets of protective immunity.Immunol Rev. 2011 Jan;239(1):8-26. doi: 10.1111/j.1600-065X.2010.00975.x. Immunol Rev. 2011. PMID: 21198662 Free PMC article. Review.
-
Functional characterization of the vaccinia virus I5 protein.Virol J. 2008 Dec 15;5:148. doi: 10.1186/1743-422X-5-148. Virol J. 2008. PMID: 19077320 Free PMC article.
References
-
- Baeuerle P A, Huttner W B. Chlorate–a potent inhibitor of protein sulfation in intact cells. Biochem Biophys Res Commun. 1986;141:870–877. - PubMed
-
- Butcher M, Raviprakash K, Ghosh H P. Acid pH induced fusion of cells by herpes simplex virus glycoproteins gB and gD. J Biol Chem. 1990;265:5862–5868. - PubMed
-
- Dallo S, Rodriguez J F, Esteban M. A 14K envelope protein of vaccinia virus with an important role in virus-host cell interactions is altered during virus persistence and determines the plaque size phenotype of the virus. Virology. 1987;159:423–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

