Morphology of the yeast endocytic pathway
- PMID: 9436999
- PMCID: PMC25237
- DOI: 10.1091/mbc.9.1.173
Morphology of the yeast endocytic pathway
Abstract
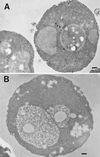
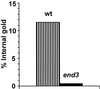
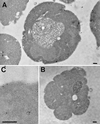
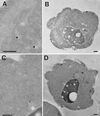
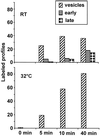
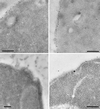
Positively charged Nanogold (Nanoprobes, Stony Brook, NY) has been developed as a new marker to follow the endocytic pathway in yeast. Positively charged Nanogold binds extensively to the surface of yeast spheroplasts and is internalized in an energy-dependent manner. Internalization of gold is blocked in the end3 mutant. During a time course of incubation of yeast spheroplasts with positively charged Nanogold at 15 degrees C, the gold was detected sequentially in small vesicles, a peripheral, vesicular/tubular compartment that we designate as an early endosome, a multivesicular body corresponding to the late endosome near the vacuole, and in the vacuole. Experiments examining endocytosis in the sec18 mutant showed an accumulation of positively charged Nanogold in approximately 30-50 nm diameter vesicles. These vesicles most likely represent the primary endocytic vesicles as no other intermediates were detected in the mutant cells, and they correspond in size to the first vesicles detected in wild-type spheroplasts at 15 degrees C. These data lend strong support to the idea that the internalization step of endocytosis in yeast involves formation of small vesicles of uniform size from the plasma membrane.
Figures


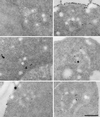
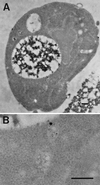
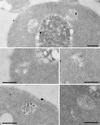
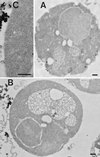
Similar articles
-
A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast.J Cell Biol. 1995 Mar;128(5):779-92. doi: 10.1083/jcb.128.5.779. J Cell Biol. 1995. PMID: 7533169 Free PMC article.
-
Ordering of compartments in the yeast endocytic pathway.Traffic. 2002 Jan;3(1):37-49. doi: 10.1034/j.1600-0854.2002.30106.x. Traffic. 2002. PMID: 11872141
-
Role of endoplasmic reticulum-derived vesicles in the formation of Golgi elements in sec23 and sec18 Saccharomyces Cerevisiae mutants.Anat Rec. 1998 Jun;251(2):256-64. doi: 10.1002/(SICI)1097-0185(199806)251:2<256::AID-AR15>3.0.CO;2-N. Anat Rec. 1998. PMID: 9624457
-
The yeast endocytic membrane transport system.Microsc Res Tech. 2000 Dec 15;51(6):547-62. doi: 10.1002/1097-0029(20001215)51:6<547::AID-JEMT5>3.0.CO;2-D. Microsc Res Tech. 2000. PMID: 11169857 Review.
-
Multivesicular bodies and multivesicular endosomes: the "ins and outs" of endosomal traffic.Sci STKE. 2002 Jul 16;2002(141):pe32. doi: 10.1126/stke.2002.141.pe32. Sci STKE. 2002. PMID: 12122203 Review.
Cited by
-
Endocytotic uptake of FITC-labeled anti-H. pylori egg yolk immunoglobulin Y in Candida yeast for detection of intracellular H. pylori.Front Microbiol. 2015 Feb 16;6:113. doi: 10.3389/fmicb.2015.00113. eCollection 2015. Front Microbiol. 2015. PMID: 25852651 Free PMC article.
-
Mechanistic mathematical model of polarity in yeast.Mol Biol Cell. 2012 May;23(10):1998-2013. doi: 10.1091/mbc.E11-10-0837. Epub 2012 Mar 21. Mol Biol Cell. 2012. PMID: 22438587 Free PMC article.
-
The p24 proteins are not essential for vesicular transport in Saccharomyces cerevisiae.Proc Natl Acad Sci U S A. 2000 Apr 11;97(8):4034-9. doi: 10.1073/pnas.070044097. Proc Natl Acad Sci U S A. 2000. PMID: 10737764 Free PMC article.
-
Endocytic delivery of intramolecularly quenched substrates and inhibitors to the intracellular yeast Kex2 protease1.Biochem J. 1999 Jul 15;341 ( Pt 2)(Pt 2):445-52. Biochem J. 1999. PMID: 10393104 Free PMC article.
-
Pep3p/Pep5p complex: a putative docking factor at multiple steps of vesicular transport to the vacuole of Saccharomyces cerevisiae.Genetics. 2000 Sep;156(1):105-22. doi: 10.1093/genetics/156.1.105. Genetics. 2000. PMID: 10978279 Free PMC article.
References
-
- Dulic V, Egerton M, Elguindi I, Raths S, Singer B, Riezman H. Yeast endocytosis assays. Methods Enzymol. 1991;194:697–710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

