Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction
- PMID: 9425011
- PMCID: PMC6792529
- DOI: 10.1523/JNEUROSCI.18-02-00687.1998
Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction
Abstract
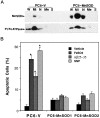
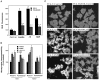
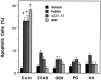
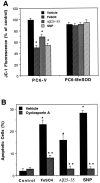
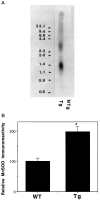
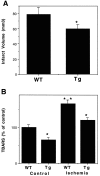
Oxidative stress is implicated in neuronal apoptosis that occurs in physiological settings and in neurodegenerative disorders. Superoxide anion radical, produced during mitochondrial respiration, is involved in the generation of several potentially damaging reactive oxygen species including peroxynitrite. To examine directly the role of superoxide and peroxynitrite in neuronal apoptosis, we generated neural cell lines and transgenic mice that overexpress human mitochondrial manganese superoxide dismutase (MnSOD). In cultured pheochromocytoma PC6 cells, overexpression of mitochondria-localized MnSOD prevented apoptosis induced by Fe2+, amyloid beta-peptide (Abeta), and nitric oxide-generating agents. Accumulations of peroxynitrite, nitrated proteins, and the membrane lipid peroxidation product 4-hydroxynonenal (HNE) after exposure to the apoptotic insults were markedly attenuated in cells expressing MnSOD. Glutathione peroxidase activity levels were increased in cells overexpressing MnSOD, suggesting a compensatory response to increased H2O2 levels. The peroxynitrite scavenger uric acid and the antioxidants propyl gallate and glutathione prevented apoptosis induced by each apoptotic insult, suggesting central roles for peroxynitrite and membrane lipid peroxidation in oxidative stress-induced apoptosis. Apoptotic insults decreased mitochondrial transmembrane potential and energy charge in control cells but not in cells overexpressing MnSOD, and cyclosporin A and caspase inhibitors protected cells against apoptosis, demonstrating roles for mitochondrial alterations and caspase activation in the apoptotic process. Membrane lipid peroxidation, protein nitration, and neuronal death after focal cerebral ischemia were significantly reduced in transgenic mice overexpressing human MnSOD. The data suggest that mitochondrial superoxide accumulation and consequent peroxynitrite production and mitochondrial dysfunction play pivotal roles in neuronal apoptosis induced by diverse insults in cell culture and in vivo.
Figures

Similar articles
-
Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration.J Neurosci Res. 1997 Sep 15;49(6):681-97. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. J Neurosci Res. 1997. PMID: 9335256
-
Hepatic mitochondrial DNA depletion after an alcohol binge in mice: probable role of peroxynitrite and modulation by manganese superoxide dismutase.J Pharmacol Exp Ther. 2010 Mar;332(3):886-97. doi: 10.1124/jpet.109.160879. Epub 2009 Dec 16. J Pharmacol Exp Ther. 2010. PMID: 20016022
-
Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury.J Neurochem. 2007 Apr;101(1):168-81. doi: 10.1111/j.1471-4159.2006.04353.x. J Neurochem. 2007. PMID: 17394464
-
MnSOD overexpression prevents liver mitochondrial DNA depletion after an alcohol binge but worsens this effect after prolonged alcohol consumption in mice.Dig Dis. 2010;28(6):756-75. doi: 10.1159/000324284. Epub 2011 Apr 27. Dig Dis. 2010. PMID: 21525761 Review.
-
Invited review: manganese superoxide dismutase in disease.Free Radic Res. 2001 Apr;34(4):325-36. doi: 10.1080/10715760100300281. Free Radic Res. 2001. PMID: 11328670 Review.
Cited by
-
Mitochondrial impairment, decreased sirtuin activity and protein acetylation in dorsal root ganglia in Friedreich Ataxia models.Cell Mol Life Sci. 2023 Dec 21;81(1):12. doi: 10.1007/s00018-023-05064-4. Cell Mol Life Sci. 2023. PMID: 38129330 Free PMC article.
-
Characterizing Sex Differences in Mitochondrial Dysfunction After Severe Traumatic Brain Injury in Mice.Neurotrauma Rep. 2023 Sep 25;4(1):627-642. doi: 10.1089/neur.2023.0046. eCollection 2023. Neurotrauma Rep. 2023. PMID: 37752924 Free PMC article.
-
Effects of tetrahydroxy stilbene glycoside derivatives on free radical damage and apoptosis in APP695V717I transgenic mice.Redox Rep. 2023 Dec;28(1):2259246. doi: 10.1080/13510002.2023.2259246. Epub 2023 Sep 20. Redox Rep. 2023. PMID: 37728223 Free PMC article.
-
Interferon Gamma Enhances Cytoprotective Pathways via Nrf2 and MnSOD Induction in Friedreich's Ataxia Cells.Int J Mol Sci. 2023 Aug 11;24(16):12687. doi: 10.3390/ijms241612687. Int J Mol Sci. 2023. PMID: 37628866 Free PMC article.
-
Aucubin Exerts Neuroprotection against Forebrain Ischemia and Reperfusion Injury in Gerbils through Antioxidative and Neurotrophic Effects.Antioxidants (Basel). 2023 May 11;12(5):1082. doi: 10.3390/antiox12051082. Antioxidants (Basel). 2023. PMID: 37237948 Free PMC article.
References
-
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors α and β protect neurons against amyloid β-peptide toxicity: evidence for involvement of a κB-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. - PMC - PubMed
-
- Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21:330–334. - PubMed
-
- Beckman J, Ye Y, Anderson P, Chen J, Accavitti M, Tarpey M, White C. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. - PubMed
-
- Behl C, Davis J, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 1994;77:817–827. - PubMed
-
- Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol Aging. 1995;16:661–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
