Antibody-dependent cellular cytotoxicity directed against cells expressing human immunodeficiency virus type 1 envelope of primary or laboratory-adapted strains by human and chimpanzee monoclonal antibodies of different epitope specificities
- PMID: 9420226
- PMCID: PMC109375
- DOI: 10.1128/JVI.72.1.286-293.1998
Antibody-dependent cellular cytotoxicity directed against cells expressing human immunodeficiency virus type 1 envelope of primary or laboratory-adapted strains by human and chimpanzee monoclonal antibodies of different epitope specificities
Abstract
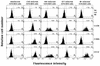
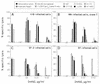
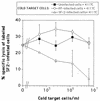
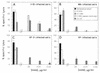
The characteristics of antibody-dependent cellular cytotoxicity (ADCC) directed by a panel of human and chimpanzee antienvelope (anti-Env) monoclonal antibodies (MAbs) of different epitope specificities were studied; this was accomplished by using target cells expressing human immunodeficiency virus type 1 (HIV-1) Envs of either primary or laboratory-adapted strains. Human MAbs of similar apparent affinities (1 x 10(9) to 2 x 10(9) liters/mol) against either a "cluster II"-overlapping epitope of gp41 or against the CD4 binding site, V3 loop, or C5 domain of gp120 directed substantial and comparable levels of specific lysis against targets infected with laboratory-adapted strains of HIV-1. As expected, those MAbs specific for relatively conserved regions of Env generally exhibited ADCC activity against a broader range of HIV-1 strains than those directed against variable epitopes. Significant ADCC activities of selected MAbs against primary isolate Env-expressing cells were demonstrated. In addition, a new ADCC epitope in the V2 domain of gp120 was defined. CD56+ cells were demonstrated to be the effector cells in these studies by fluorescence-activated cell sorting followed by ADCC assays. Notably, all anti-Env MAbs tested in this study, including MAbs directed against each of the known neutralization epitope clusters in gp120, directed significant levels of ADCC against targets expressing Env of one or more HIV-1 strains. These results imply that many, if not most, HIV-1-neutralizing human Abs of high affinity (> or = 3 x 10(8) liters/mol in these studies) and of the immunoglobulin G1 (IgG1) subclass (i.e., the predominate IgG subclass) are capable of directing ADCC. Since neutralizing Abs have been associated with long-term survival following HIV-1 infection, this suggests that ADCC activity may be beneficial in vivo.
Figures

Similar articles
-
A novel antibody-dependent cellular cytotoxicity epitope in gp120 is identified by two monoclonal antibodies isolated from a long-term survivor of human immunodeficiency virus type 1 infection.J Virol. 1997 Feb;71(2):925-33. doi: 10.1128/JVI.71.2.925-933.1997. J Virol. 1997. PMID: 8995609 Free PMC article.
-
The C108g epitope in the V2 domain of gp120 functions as a potent neutralization target when introduced into envelope proteins derived from human immunodeficiency virus type 1 primary isolates.J Virol. 2005 Jun;79(11):6909-17. doi: 10.1128/JVI.79.11.6909-6917.2005. J Virol. 2005. PMID: 15890930 Free PMC article.
-
Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities.J Virol. 1994 May;68(5):3015-26. doi: 10.1128/JVI.68.5.3015-3026.1994. J Virol. 1994. PMID: 7512157 Free PMC article.
-
Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses.Curr HIV Res. 2013 Jul;11(5):378-87. doi: 10.2174/1570162x113116660059. Curr HIV Res. 2013. PMID: 24191939 Free PMC article. Review.
-
Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates.Expert Rev Vaccines. 2006 Jun;5(3):347-63. doi: 10.1586/14760584.5.3.347. Expert Rev Vaccines. 2006. PMID: 16827619 Free PMC article. Review.
Cited by
-
Pol as a target for antibody dependent cellular cytotoxicity responses in HIV-1 infection.Virology. 2011 Mar 30;412(1):110-6. doi: 10.1016/j.virol.2010.12.044. Epub 2011 Jan 26. Virology. 2011. PMID: 21269655 Free PMC article.
-
Passively Acquired Constant Region 5-Specific Antibodies Associated With Improved Survival in Infants Who Acquire Human Immunodeficiency Virus.Open Forum Infect Dis. 2023 Jun 14;10(7):ofad316. doi: 10.1093/ofid/ofad316. eCollection 2023 Jul. Open Forum Infect Dis. 2023. PMID: 37426948 Free PMC article.
-
Cocrystal Structures of Antibody N60-i3 and Antibody JR4 in Complex with gp120 Define More Cluster A Epitopes Involved in Effective Antibody-Dependent Effector Function against HIV-1.J Virol. 2015 Sep;89(17):8840-54. doi: 10.1128/JVI.01232-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085162 Free PMC article.
-
Antibody-Dependent Cellular Cytotoxicity and NK Cell-Driven Immune Escape in HIV Infection: Implications for HIV Vaccine Development.Adv Virol. 2012;2012:637208. doi: 10.1155/2012/637208. Epub 2012 Apr 29. Adv Virol. 2012. PMID: 22611395 Free PMC article.
-
Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection.J Clin Immunol. 2001 May;21(3):227-33. doi: 10.1023/a:1011087132180. J Clin Immunol. 2001. PMID: 11403230
References
-
- Alsmadi, O., and S. A. Tilley. Unpublished data.
-
- Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. - PubMed
-
- Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. New Engl J Med. 1995;332:201–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

