Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s
- PMID: 9419219
- PMCID: PMC2199181
- DOI: 10.1084/jem.187.1.129
Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s
Abstract
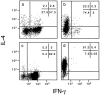
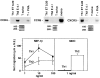
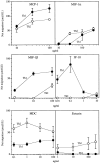
T helper cells type 1 (Th1s) that produce interferon-gamma predominantly mediate cellular immune responses and are involved in the development of chronic inflammatory conditions, whereas Th2s which produce large amounts of IL-4 and IL-5 upregulate IgE production and are prominent in the pathogenesis of allergic diseases. The precise factors determining whether Th1- or Th2-mediated immune responses preferentially occur at a peripheral site of antigen exposure are largely unknown. Chemokines, a superfamily of polypeptide mediators, are a key component of the leukocyte recruitment process. Here we report that among four CXC (CXCR1-4) and five CC (CCR1-5) chemokine receptors analyzed, CXCR3 and CCR5 are preferentially expressed in human Th1s. In contrast, Th2s preferentially express CCR4 and, to a lesser extent, CCR3. In agreement with the differential chemokine receptor expression, Th1s and Th2s selectively migrate in response to the corresponding chemokines. The differential expression of chemokine receptors may dictate, to a large extent, the migration and tissue homing of Th1s and Th2s. It may also determine different susceptibility of Th1s and Th2s to human immunodeficiency virus strains using different fusion coreceptors.
Figures


Similar articles
-
Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes.J Exp Med. 1998 Mar 16;187(6):875-83. doi: 10.1084/jem.187.6.875. J Exp Med. 1998. PMID: 9500790 Free PMC article.
-
Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity.J Immunol. 2001 Jan 15;166(2):996-1002. doi: 10.4049/jimmunol.166.2.996. J Immunol. 2001. PMID: 11145678
-
CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions.Proc Natl Acad Sci U S A. 1999 Jun 8;96(12):6873-8. doi: 10.1073/pnas.96.12.6873. Proc Natl Acad Sci U S A. 1999. PMID: 10359806 Free PMC article.
-
T cell chemokine receptor expression in human Th1- and Th2-associated diseases.Arch Immunol Ther Exp (Warsz). 2000;48(6):451-6. Arch Immunol Ther Exp (Warsz). 2000. PMID: 11197598 Review.
-
[The roles of cytokine receptors in diseases].Rinsho Byori. 2000 May;48(5):409-15. Rinsho Byori. 2000. PMID: 10892288 Review. Japanese.
Cited by
-
CXCL9/Mig mediates T cells recruitment to valvular tissue lesions of chronic rheumatic heart disease patients.Inflammation. 2013 Aug;36(4):800-11. doi: 10.1007/s10753-013-9606-2. Inflammation. 2013. PMID: 23417848 Free PMC article.
-
Modulation of Immune Response to Chlamydia muridarum by Host miR-135a.Front Cell Infect Microbiol. 2021 Apr 13;11:638058. doi: 10.3389/fcimb.2021.638058. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33928045 Free PMC article.
-
Cytokine/chemokine profiles contribute to understanding the pathogenesis and diagnosis of primary Sjögren's syndrome.Clin Exp Immunol. 2012 Jul;169(1):17-26. doi: 10.1111/j.1365-2249.2012.04587.x. Clin Exp Immunol. 2012. PMID: 22670774 Free PMC article.
-
Virus-Driven Carcinogenesis.Cancers (Basel). 2021 May 27;13(11):2625. doi: 10.3390/cancers13112625. Cancers (Basel). 2021. PMID: 34071792 Free PMC article. Review.
-
Inhibition of G-Protein βγ Signaling Decreases Levels of Messenger RNAs Encoding Proinflammatory Cytokines in T Cell Receptor-Stimulated CD4(+) T Helper Cells.J Mol Signal. 2015 Jul 6;10:1. doi: 10.5334/1750-2187-10-1. J Mol Signal. 2015. PMID: 27095999 Free PMC article.
References
-
- Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
-
- Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. - PubMed
-
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

