The yeast HPR1 gene has a functional role in transcriptional elongation that uncovers a novel source of genome instability
- PMID: 9407037
- PMCID: PMC316820
- DOI: 10.1101/gad.11.24.3459
The yeast HPR1 gene has a functional role in transcriptional elongation that uncovers a novel source of genome instability
Abstract
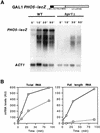
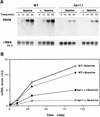
The yeast HPR1 gene plays an important role in genome stability, as indicated by the observation that hpr1 mutants have high frequencies of DNA repeat recombination and chromosome loss. Here we report that HPR1 is required for transcriptional elongation. Transcription driven from constitutive and regulated yeast promoters cannot elongate through the bacterial lacZ coding region in hpr1Delta cells, but progresses efficiently through other sequences such as yeast PHO5. We show that HPR1 is not required for transcription activation and that the previously reported effects of hpr1Delta on the activation of different promoters is a consequence of the incapacity of hpr1Delta cells to elongate transcription through lacZ, used as reporter. Transcriptional defects are also observed in yeast DNA sequences of hpr1Delta cells in the presence of the transcription elongation inhibitor 6-azauracil. In all cases, the blockage of transcription elongation in hpr1Delta is associated with both the high frequency of deletions and the increase in plasmid instability that we report here. Therefore, in addition to the identification of a new element involved in transcriptional elongation, our work provides evidence for a new source of genomic instability.
Figures







Similar articles
-
Recombination between DNA repeats in yeast hpr1delta cells is linked to transcription elongation.EMBO J. 1997 May 15;16(10):2826-35. doi: 10.1093/emboj/16.10.2826. EMBO J. 1997. PMID: 9184227 Free PMC article.
-
A novel yeast gene, THO2, is involved in RNA pol II transcription and provides new evidence for transcriptional elongation-associated recombination.EMBO J. 1998 Aug 17;17(16):4859-72. doi: 10.1093/emboj/17.16.4859. EMBO J. 1998. PMID: 9707445 Free PMC article.
-
A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae.EMBO J. 2000 Nov 1;19(21):5824-34. doi: 10.1093/emboj/19.21.5824. EMBO J. 2000. PMID: 11060033 Free PMC article.
-
Recombination proteins in yeast.Annu Rev Genet. 2004;38:233-71. doi: 10.1146/annurev.genet.38.072902.091500. Annu Rev Genet. 2004. PMID: 15568977 Review.
-
Transcription and chromatin converge: lessons from yeast genetics.Curr Opin Genet Dev. 2001 Apr;11(2):142-7. doi: 10.1016/s0959-437x(00)00171-4. Curr Opin Genet Dev. 2001. PMID: 11250136 Review.
Cited by
-
Transcription-replication conflicts at chromosomal fragile sites-consequences in M phase and beyond.Chromosoma. 2017 Mar;126(2):213-222. doi: 10.1007/s00412-016-0617-2. Epub 2016 Oct 28. Chromosoma. 2017. PMID: 27796495 Review.
-
The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability.EMBO J. 2002 Jul 1;21(13):3526-35. doi: 10.1093/emboj/cdf335. EMBO J. 2002. PMID: 12093753 Free PMC article.
-
An early function during transcription for the yeast mRNA export factor Dbp5p/Rat8p suggested by its genetic and physical interactions with transcription factor IIH components.Mol Biol Cell. 2003 Apr;14(4):1664-76. doi: 10.1091/mbc.e02-09-0602. Mol Biol Cell. 2003. PMID: 12686617 Free PMC article.
-
Genome-wide mRNA surveillance is coupled to mRNA export.Genes Dev. 2004 Nov 1;18(21):2652-62. doi: 10.1101/gad.1241204. Epub 2004 Oct 15. Genes Dev. 2004. PMID: 15489286 Free PMC article.
-
Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II.Mol Cell Biol. 2003 Jun;23(12):4207-18. doi: 10.1128/MCB.23.12.4207-4218.2003. Mol Cell Biol. 2003. PMID: 12773564 Free PMC article.
References
-
- Aso T, Lane WS, Conaway JW, Conaway RC. Elongin (SIII): A multisubunit regulator of elongation by RNA polymerase II. Science. 1995;269:1439–1443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
