Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides
- PMID: 9405632
- PMCID: PMC25016
- DOI: 10.1073/pnas.94.26.14444
Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides
Abstract
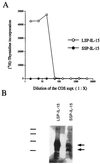
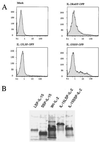
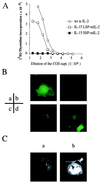
Two isoforms of human interleukin 15 (IL-15) exist. One isoform has a shorter putative signal peptide (21 amino acids) and its transcript shows a tissue distribution pattern that is distinct from that of the alternative IL-15 isoform with a 48-aa signal peptide. The 21-aa signal isoform is preferentially expressed in tissues such as testis and thymus. Experiments using different combinations of signal peptides and mature proteins (IL-2, IL-15, and green fluorescent protein) showed that the short signal peptide regulates the fate of the mature protein by controlling the intracellular trafficking to nonendoplasmic reticulum sites, whereas the long signal peptide both regulates the rate of protein translation and functions as a secretory signal peptide. As a consequence, the IL-15 associated with the short signal peptide is not secreted, but rather is stored intracellularly, appearing in the nucleus and cytoplasmic components. Such production of an intracellular lymphokine is not typical of other soluble interleukin systems, suggesting a biological function for IL-15 as an intracellular molecule.
Figures


Similar articles
-
The long signal peptide isoform and its alternative processing direct the intracellular trafficking of interleukin-15.J Biol Chem. 2000 Sep 29;275(39):30653-9. doi: 10.1074/jbc.M002373200. J Biol Chem. 2000. PMID: 10869346
-
A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing.FASEB J. 2005 Jan;19(1):19-28. doi: 10.1096/fj.04-2633com. FASEB J. 2005. PMID: 15629891
-
Expression of two interleukin-15 mRNA isoforms in human tumors does not correlate with secretion: role of different signal peptides.Eur J Immunol. 1997 May;27(5):1049-54. doi: 10.1002/eji.1830270502. Eur J Immunol. 1997. PMID: 9174591
-
The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens.Annu Rev Immunol. 1999;17:19-49. doi: 10.1146/annurev.immunol.17.1.19. Annu Rev Immunol. 1999. PMID: 10358752 Review.
-
Intracellular interleukin (IL)-1 family cytokine processing enzyme.Arch Pharm Res. 2016 Nov;39(11):1556-1564. doi: 10.1007/s12272-016-0855-0. Epub 2016 Nov 9. Arch Pharm Res. 2016. PMID: 27826753 Review.
Cited by
-
Characterization and favorable in vivo properties of heterodimeric soluble IL-15·IL-15Rα cytokine compared to IL-15 monomer.J Biol Chem. 2013 Jun 21;288(25):18093-103. doi: 10.1074/jbc.M113.461756. Epub 2013 May 6. J Biol Chem. 2013. PMID: 23649624 Free PMC article.
-
Regulating the immune system via IL-15 transpresentation.Cytokine. 2012 Sep;59(3):479-90. doi: 10.1016/j.cyto.2012.06.017. Epub 2012 Jul 12. Cytokine. 2012. PMID: 22795955 Free PMC article. Review.
-
Differential roles of interleukin 15 mRNA isoforms generated by alternative splicing in immune responses in vivo.J Exp Med. 2000 Jan 3;191(1):157-70. doi: 10.1084/jem.191.1.157. J Exp Med. 2000. PMID: 10620614 Free PMC article.
-
The activation of TLR7 regulates the expression of VEGF, TIMP1, MMP2, IL-6, and IL-15 in Hela cells.Mol Cell Biochem. 2014 Apr;389(1-2):43-9. doi: 10.1007/s11010-013-1925-y. Epub 2013 Dec 18. Mol Cell Biochem. 2014. PMID: 24347177
-
Molecular pathways: interleukin-15 signaling in health and in cancer.Clin Cancer Res. 2014 Apr 15;20(8):2044-50. doi: 10.1158/1078-0432.CCR-12-3603. Clin Cancer Res. 2014. PMID: 24737791 Free PMC article. Review.
References
-
- Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn A, Ahdieh M, Johnson L, Alderson M R, Watson J D, Anderson D M, Giri J G. Science. 1994;264:965–968. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

