Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase
- PMID: 9391141
- PMCID: PMC28421
- DOI: 10.1073/pnas.94.25.13997
Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase
Abstract
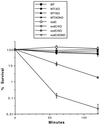
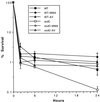
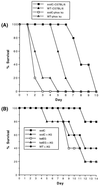
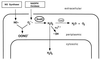
Superoxide dismutase (SOD) catalyzes the conversion of superoxide radical to hydrogen peroxide. Periplasmic localization of bacterial Cu,Zn-SOD has suggested a role of this enzyme in defense against extracellular phagocyte-derived reactive oxygen species. Sequence analysis of regions flanking the Salmonella typhimurium sodC gene encoding Cu,Zn-SOD demonstrates significant homology to lambda phage proteins, reflecting possible bacteriophage-mediated horizontal gene transfer of this determinant among pathogenic bacteria. Salmonella deficient in Cu,Zn-SOD has reduced survival in macrophages and attenuated virulence in mice, which can be restored by abrogation of either the phagocyte respiratory burst or inducible nitric oxide synthase. Moreover, a sodC mutant is extremely susceptible to the combination of superoxide and nitric oxide. These observations suggest that SOD protects periplasmic or inner membrane targets by diverting superoxide and limiting peroxynitrite formation, and they demonstrate the ability of the respiratory burst and nitric oxide synthase to synergistically kill microbial pathogens in vivo.
Figures


Similar articles
-
Cu,Zn superoxide dismutase of Mycobacterium tuberculosis contributes to survival in activated macrophages that are generating an oxidative burst.Infect Immun. 2001 Aug;69(8):4980-7. doi: 10.1128/IAI.69.8.4980-4987.2001. Infect Immun. 2001. PMID: 11447176 Free PMC article.
-
Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis.Mol Microbiol. 1997 Aug;25(4):785-96. doi: 10.1046/j.1365-2958.1997.5151877.x. Mol Microbiol. 1997. PMID: 9379906
-
Phagocytic superoxide specifically damages an extracytoplasmic target to inhibit or kill Salmonella.PLoS One. 2009;4(3):e4975. doi: 10.1371/journal.pone.0004975. Epub 2009 Mar 23. PLoS One. 2009. PMID: 19305502 Free PMC article.
-
Salmonella evasion of the NADPH phagocyte oxidase.Microbes Infect. 2001 Nov-Dec;3(14-15):1313-20. doi: 10.1016/s1286-4579(01)01492-7. Microbes Infect. 2001. PMID: 11755420 Review.
-
Chemical Warfare at the Microorganismal Level: A Closer Look at the Superoxide Dismutase Enzymes of Pathogens.ACS Infect Dis. 2018 Jun 8;4(6):893-903. doi: 10.1021/acsinfecdis.8b00026. Epub 2018 Mar 14. ACS Infect Dis. 2018. PMID: 29517910 Free PMC article. Review.
Cited by
-
Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion.Microbiol Mol Biol Rev. 2004 Sep;68(3):560-602, table of contents. doi: 10.1128/MMBR.68.3.560-602.2004. Microbiol Mol Biol Rev. 2004. PMID: 15353570 Free PMC article. Review.
-
Steps toward broad-spectrum therapeutics: discovering virulence-associated genes present in diverse human pathogens.BMC Genomics. 2009 Oct 29;10:501. doi: 10.1186/1471-2164-10-501. BMC Genomics. 2009. PMID: 19874620 Free PMC article.
-
Role of the RecBCD recombination pathway in Salmonella virulence.J Bacteriol. 2002 Jan;184(2):592-5. doi: 10.1128/JB.184.2.592-595.2002. J Bacteriol. 2002. PMID: 11751841 Free PMC article.
-
Manganese Utilization in Salmonella Pathogenesis: Beyond the Canonical Antioxidant Response.Front Cell Dev Biol. 2022 Jul 12;10:924925. doi: 10.3389/fcell.2022.924925. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35903545 Free PMC article. Review.
-
Resistance to peroxynitrite in Neisseria gonorrhoeae.Microbiology (Reading). 2009 Aug;155(Pt 8):2532-2545. doi: 10.1099/mic.0.028092-0. Epub 2009 Apr 30. Microbiology (Reading). 2009. PMID: 19406894 Free PMC article.
References
-
- McCord J M, Fridovich I. J Biol Chem. 1969;244:6049–6055. - PubMed
-
- Puget K, Michelson A M. Biochem Biophys Res Commun. 1974;58:830–838. - PubMed
-
- Kroll J S, Langford P R, Wilks K E, Keil A D. Microbiology. 1995;141:2271–2279. - PubMed
-
- Canvin J, Langford P R, Wilks K E, Kroll J S. FEMS Microbiol Lett. 1996;136:215–220. - PubMed
-
- Benov L T, Fridovich I. J Biol Chem. 1994;269:25310–25314. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

