The beta-amyloid precursor protein of Alzheimer's disease enhances neuron viability and modulates neuronal polarity
- PMID: 9390996
- PMCID: PMC6573428
- DOI: 10.1523/JNEUROSCI.17-24-09407.1997
The beta-amyloid precursor protein of Alzheimer's disease enhances neuron viability and modulates neuronal polarity
Abstract
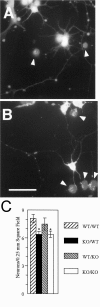
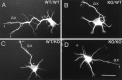
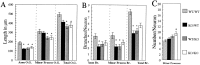
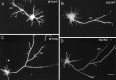
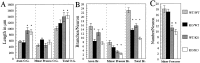
beta-Amyloid precursor protein (betaPP) can reside at neuron and glial cell surfaces or undergo proteolytic processing into secreted fragments. Although betaPP has been studied extensively, its precise physiological role is unknown. A line of transgenic knock-out mice selectively deficient in betaPP survive and breed but exhibit motor dysfunction and brain gliosis, consistent with a physiological role for betaPP in neuron development. To elucidate these functions, we cultured hippocampal neurons from wild-type and betaPP-deficient mice and compared their ability to attach, survive, and develop neurites. We found that hippocampal neurons from betaPP-deficient mice had diminished viability and retarded neurite development. We also compared the effects of betaPP secretory products, released from wild-type astrocytes, on process outgrowth from wild-type and betaPP-deficient hippocampal neurons. Outgrowth was enhanced at 1 d in the presence of wild-type astrocytes, as compared with betaPP-deficient astrocytes. However, by 3 d, neurons had shorter axons but more minor processes with more branching when cocultured with wild-type astrocytes, as compared with betaPP-deficient astrocytes. Our data demonstrate that cell-associated neuronal betaPP contributes to neuron viability, axonogenesis, and arborization and that betaPP secretory products modulate axon growth, dendrite branching, and dendrite numbers.
Figures
Similar articles
-
Knockout of Amyloid β Protein Precursor (APP) Expression Alters Synaptogenesis, Neurite Branching and Axonal Morphology of Hippocampal Neurons.Neurochem Res. 2019 Jun;44(6):1346-1355. doi: 10.1007/s11064-018-2512-0. Epub 2018 Mar 23. Neurochem Res. 2019. PMID: 29572646
-
Astrocytes down-regulate neuronal beta-amyloid precursor protein expression and modify its processing in an apolipoprotein E isoform-specific manner.Eur J Neurosci. 2001 Jul;14(2):256-66. doi: 10.1046/j.0953-816x.2001.01643.x. Eur J Neurosci. 2001. PMID: 11553277
-
Secretion of nerve growth factor from septum stimulates neurite outgrowth and release of the amyloid protein precursor of Alzheimer's disease from hippocampal explants.J Neurosci Res. 1994 Jun 15;38(3):248-58. doi: 10.1002/jnr.490380303. J Neurosci Res. 1994. PMID: 7932862
-
Transgenic mouse models of Alzheimer's disease.Ann N Y Acad Sci. 2000 Jun;908:260-6. doi: 10.1111/j.1749-6632.2000.tb06653.x. Ann N Y Acad Sci. 2000. PMID: 10911965 Review.
-
Neuronal polarity: demarcation, growth and commitment.Curr Opin Cell Biol. 2012 Aug;24(4):547-53. doi: 10.1016/j.ceb.2012.05.011. Epub 2012 Jun 20. Curr Opin Cell Biol. 2012. PMID: 22726583 Free PMC article. Review.
Cited by
-
Presenilins and APP in neuritic and synaptic plasticity: implications for the pathogenesis of Alzheimer's disease.Neuromolecular Med. 2002;2(2):167-96. doi: 10.1385/NMM:2:2:167. Neuromolecular Med. 2002. PMID: 12428810 Review.
-
Dentate gyrus volume is reduced before onset of plaque formation in PDAPP mice: a magnetic resonance microscopy and stereologic analysis.Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1381-6. doi: 10.1073/pnas.242746599. Epub 2003 Jan 24. Proc Natl Acad Sci U S A. 2003. PMID: 12552120 Free PMC article.
-
The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement.J Cell Biol. 2001 Jun 25;153(7):1403-14. doi: 10.1083/jcb.153.7.1403. J Cell Biol. 2001. PMID: 11425871 Free PMC article.
-
Amyloid Precursor Protein Dimerisation Reduces Neurite Outgrowth.Mol Neurobiol. 2019 Jan;56(1):13-28. doi: 10.1007/s12035-018-1070-4. Epub 2018 Apr 19. Mol Neurobiol. 2019. PMID: 29675574
-
The endocrine dyscrasia that accompanies menopause and andropause induces aberrant cell cycle signaling that triggers re-entry of post-mitotic neurons into the cell cycle, neurodysfunction, neurodegeneration and cognitive disease.Horm Behav. 2015 Nov;76:63-80. doi: 10.1016/j.yhbeh.2015.06.021. Epub 2015 Jul 16. Horm Behav. 2015. PMID: 26188949 Free PMC article. Review.
References
-
- Banker G, Goslin K. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT; Cambridge, MA: 1991. pp. 251–281.
-
- Brandt R, Lee G. Functional organization of microtubule-associated protein tau. J Biol Chem. 1993;268:3414–3419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
