The mammalian protein (rbet1) homologous to yeast Bet1p is primarily associated with the pre-Golgi intermediate compartment and is involved in vesicular transport from the endoplasmic reticulum to the Golgi apparatus
- PMID: 9382863
- PMCID: PMC2140212
- DOI: 10.1083/jcb.139.5.1157
The mammalian protein (rbet1) homologous to yeast Bet1p is primarily associated with the pre-Golgi intermediate compartment and is involved in vesicular transport from the endoplasmic reticulum to the Golgi apparatus
Abstract
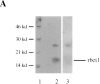
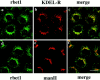
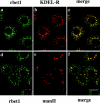
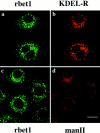
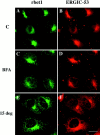
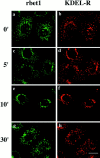
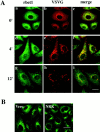
Yeast Bet1p participates in vesicular transport from the endoplasmic reticulum to the Golgi apparatus and functions as a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) associated with ER-derived vesicles. A mammalian protein (rbet1) homologous to Bet1p was recently identified, and it was concluded that rbet1 is associated with the Golgi apparatus based on the subcellular localization of transiently expressed epitope-tagged rbet1. In the present study using rabbit antibodies raised against the cytoplasmic domain of rbet1, we found that the majority of rbet1 is not associated with the Golgi apparatus as marked by the Golgi mannosidase II in normal rat kidney cells. Rather, rbet1 is predominantly associated with vesicular spotty structures that concentrate in the peri-Golgi region but are also present throughout the cytoplasm. These structures colocalize with the KDEL receptor and ERGIC-53, which are known to be enriched in the intermediate compartment. When the Golgi apparatus is fragmented by nocodazole treatment, a significant portion of rbet1 is not colocalized with structures marked by Golgi mannosidase II or the KDEL receptor. Association of rbet1 in cytoplasmic spotty structures is apparently not altered by preincubation of cells at 15 degrees C. However, upon warming up from 15 to 37 degrees C, rbet1 concentrates into the peri-Golgi region. Furthermore, rbet1 colocalizes with vesicular stomatitis virus G-protein en route from the ER to the Golgi. Antibodies against rbet1 inhibit in vitro transport of G-protein from the ER to the Golgi apparatus in a dose-dependent manner. This inhibition can be neutralized by preincubation of antibodies with recombinant rbet1. EGTA is known to inhibit ER-Golgi transport at a stage after vesicle docking but before the actual fusion event. Antibodies against rbet1 inhibit ER-Golgi transport only when they are added before the EGTA-sensitive stage. These results suggest that rbet1 may be involved in the docking process of ER-derived vesicles with the cis-Golgi membrane.
Figures






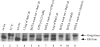
Similar articles
-
Stx5-Mediated ER-Golgi Transport in Mammals and Yeast.Cells. 2019 Jul 26;8(8):780. doi: 10.3390/cells8080780. Cells. 2019. PMID: 31357511 Free PMC article. Review.
-
Morphological and functional association of Sec22b/ERS-24 with the pre-Golgi intermediate compartment.Mol Biol Cell. 1999 Feb;10(2):435-53. doi: 10.1091/mbc.10.2.435. Mol Biol Cell. 1999. PMID: 9950687 Free PMC article.
-
Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport.J Biol Chem. 2001 Jul 20;276(29):27480-7. doi: 10.1074/jbc.M102786200. Epub 2001 Apr 25. J Biol Chem. 2001. PMID: 11323436
-
Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs.J Cell Biol. 1998 Jun 29;141(7):1489-502. doi: 10.1083/jcb.141.7.1489. J Cell Biol. 1998. PMID: 9647643 Free PMC article.
-
SNAREs and membrane fusion in the Golgi apparatus.Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review.
Cited by
-
GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus.Mol Biol Cell. 2002 Oct;13(10):3493-507. doi: 10.1091/mbc.e02-01-0004. Mol Biol Cell. 2002. PMID: 12388752 Free PMC article.
-
Countercurrent distribution of two distinct SNARE complexes mediating transport within the Golgi stack.Mol Biol Cell. 2004 Apr;15(4):1506-18. doi: 10.1091/mbc.e03-08-0625. Epub 2004 Jan 23. Mol Biol Cell. 2004. PMID: 14742712 Free PMC article.
-
Congenital disorder of glycosylation caused by starting site-specific variant in syntaxin-5.Nat Commun. 2021 Oct 28;12(1):6227. doi: 10.1038/s41467-021-26534-y. Nat Commun. 2021. PMID: 34711829 Free PMC article.
-
Stx5-Mediated ER-Golgi Transport in Mammals and Yeast.Cells. 2019 Jul 26;8(8):780. doi: 10.3390/cells8080780. Cells. 2019. PMID: 31357511 Free PMC article. Review.
-
Sugary Logistics Gone Wrong: Membrane Trafficking and Congenital Disorders of Glycosylation.Int J Mol Sci. 2020 Jun 30;21(13):4654. doi: 10.3390/ijms21134654. Int J Mol Sci. 2020. PMID: 32629928 Free PMC article. Review.
References
-
- Balch WE, McCaffery JM, Plunter H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;77:841–852. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

