The human DnaJ homologue dj2 facilitates mitochondrial protein import and luciferase refolding
- PMID: 9382858
- PMCID: PMC2140199
- DOI: 10.1083/jcb.139.5.1089
The human DnaJ homologue dj2 facilitates mitochondrial protein import and luciferase refolding
Abstract
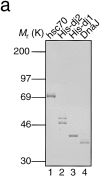
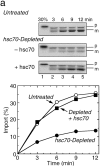
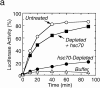
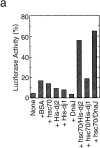
DnaJ homologues function in cooperation with hsp70 family members in various cellular processes including intracellular protein trafficking and folding. Three human DnaJ homologues present in the cytosol have been identified: dj1 (hsp40/hdj-1), dj2 (HSDJ/hdj-2), and neuronal tissue-specific hsj1. dj1 is thought to be engaged in folding of nascent polypeptides, whereas functions of the other DnaJ homologues remain to be elucidated. To investigate roles of dj2 and dj1, we developed a system of chaperone depletion from and readdition to rabbit reticulocyte lysates. Using this system, we found that heat shock cognate 70 protein (hsc70) and dj2, but not dj1, are involved in mitochondrial import of preornithine transcarbamylase. Bacterial DnaJ could replace mammalian dj2 in mitochondrial protein import. We also tested the effects of these DnaJ homologues on folding of guanidine-denatured firefly luciferase. Unexpectedly, dj2, but not dj1, together with hsc70 refolded the protein efficiently. We propose that dj2 is the functional partner DnaJ homologue of hsc70 in the mammalian cytosol. Bacterial DnaJ protein could replace mammalian dj2 in the refolding of luciferase. Thus, the cytosolic chaperone system for mitochondrial protein import and for protein folding is highly conserved, involving DnaK and DnaJ in bacteria, Ssa1-4p and Ydj1p in yeast, and hsc70 and dj2 in mammals.
Figures









Similar articles
-
Human DnaJ homologs dj2 and dj3, and bag-1 are positive cochaperones of hsc70.J Biol Chem. 2000 Aug 11;275(32):24728-34. doi: 10.1074/jbc.M002021200. J Biol Chem. 2000. PMID: 10816573
-
hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria.Cell Death Differ. 2004 Apr;11(4):390-402. doi: 10.1038/sj.cdd.4401369. Cell Death Differ. 2004. PMID: 14752510
-
Hsc70/Hsp40 chaperone system mediates the Hsp90-dependent refolding of firefly luciferase.Genes Cells. 1999 Dec;4(12):721-9. doi: 10.1046/j.1365-2443.1999.00299.x. Genes Cells. 1999. PMID: 10620017
-
Molecular chaperones as essential mediators of mitochondrial biogenesis.Biochim Biophys Acta. 2002 Sep 2;1592(1):51-62. doi: 10.1016/s0167-4889(02)00264-1. Biochim Biophys Acta. 2002. PMID: 12191768 Review.
-
DnaJ-like proteins: molecular chaperones and specific regulators of Hsp70.Trends Biochem Sci. 1994 Apr;19(4):176-81. doi: 10.1016/0968-0004(94)90281-x. Trends Biochem Sci. 1994. PMID: 8016869 Review.
Cited by
-
An RNA aptamer specific to Hsp70-ATP conformation inhibits its ATPase activity independent of Hsp40.Nucleic Acid Ther. 2015 Apr;25(2):103-12. doi: 10.1089/nat.2014.0510. Epub 2015 Feb 5. Nucleic Acid Ther. 2015. PMID: 25654640 Free PMC article.
-
Structure and function of human DnaJ homologue subfamily a member 1 (DNAJA1) and its relationship to pancreatic cancer.Biochemistry. 2014 Mar 4;53(8):1360-72. doi: 10.1021/bi401329a. Epub 2014 Feb 19. Biochemistry. 2014. PMID: 24512202 Free PMC article.
-
SGT2 and MDY2 interact with molecular chaperone YDJ1 in Saccharomyces cerevisiae.Cell Stress Chaperones. 2007 Spring;12(1):59-70. doi: 10.1379/csc-220r.1. Cell Stress Chaperones. 2007. PMID: 17441508 Free PMC article.
-
Different Transcriptomic Responses to Thermal Stress in Heat-Tolerant and Heat-Sensitive Pacific Abalones Indicated by Cardiac Performance.Front Physiol. 2019 Jan 9;9:1895. doi: 10.3389/fphys.2018.01895. eCollection 2018. Front Physiol. 2019. PMID: 30687115 Free PMC article.
-
Increased levels of mitochondrial import factor Mia40 prevent the aggregation of polyQ proteins in the cytosol.EMBO J. 2021 Aug 16;40(16):e107913. doi: 10.15252/embj.2021107913. Epub 2021 Jun 30. EMBO J. 2021. PMID: 34191328 Free PMC article.
References
-
- Bukau B, Hesterkamp T, Luirink J. Growing up in a dangerous environment: a network of multiple targeting and folding pathways for nascent polypeptides in the cytosol. Trends Cell Biol. 1996;6:480–486. - PubMed
-
- Caplan AJ, Tsai J, Casey PJ, Douglas MG. Farnesylation of YDJ1p is required for function at elevated growth temperatures in Saccharomyces cerevisiae. . J Biol Chem. 1992a;267:18890–18895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

