An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains
- PMID: 9380744
- PMCID: PMC23549
- DOI: 10.1073/pnas.94.20.10973
An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains
Abstract
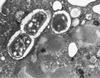
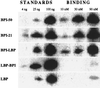
The host response to Gram-negative bacterial infection is influenced by two homologous lipopolysaccharide (LPS)-interactive proteins, LPS-binding protein (LBP) and the bacteridical/permeability-increasing protein (BPI). Both proteins bind LPS via their N-terminal domains but produce profoundly different effects: BPI and a bioactive N-terminal fragment BPI-21 exert a selective and potent antibacterial effect upon Gram-negative bacteria and suppress LPS bioactivity whereas LBP is not toxic toward Gram-negative bacteria and potentiates LPS bioactivity. The latter effect of LBP requires the C-terminal domain for delivery of LPS to CD14, so we postulated that the C-terminal region of BPI may serve a similar delivery function but to distinct targets. LBP, holoBPI, BPI-21, and LBP/BPI chimeras were compared for their ability to promote uptake by human phagocytes of an encapsulated, phagocytosis-resistant strain of Escherichia coli. We show that only bacteria preincubated with holoBPI are ingested by neutrophils and monocytes. These findings suggest that, when extracellular holoBPI is bound via its N-terminal domain to Gram-negative bacteria, the C-terminal domain promotes bacterial attachment to neutrophils and monocytes, leading to phagocytosis. Therefore, analogous to the role of the C-terminal domain of LBP in delivery of LPS to CD14, the C-terminal domain of BPI may fulfill a similar function in BPI-specific disposal pathways for Gram-negative bacteria.
Figures
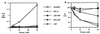

Similar articles
-
Human bactericidal/permeability-increasing protein and a recombinant NH2-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit tumor necrosis factor release induced by the bacteria.J Clin Invest. 1992 Sep;90(3):1122-30. doi: 10.1172/JCI115930. J Clin Invest. 1992. PMID: 1522221 Free PMC article.
-
Endotoxin-neutralizing properties of the 25 kD N-terminal fragment and a newly isolated 30 kD C-terminal fragment of the 55-60 kD bactericidal/permeability-increasing protein of human neutrophils.J Exp Med. 1991 Sep 1;174(3):649-55. doi: 10.1084/jem.174.3.649. J Exp Med. 1991. PMID: 1875165 Free PMC article.
-
Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli.Infect Immun. 1994 Jan;62(1):259-65. doi: 10.1128/iai.62.1.259-265.1994. Infect Immun. 1994. PMID: 8262637 Free PMC article.
-
The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide.Immunobiology. 1993 Apr;187(3-5):417-29. doi: 10.1016/S0171-2985(11)80354-2. Immunobiology. 1993. PMID: 8330906 Review.
-
Bactericidal permeability-increasing protein in host defence against gram-negative bacteria and endotoxin.Ciba Found Symp. 1994;186:176-87; discussion 187-9. doi: 10.1002/9780470514658.ch11. Ciba Found Symp. 1994. PMID: 7768151 Review.
Cited by
-
Anti-neutrophil cytoplasmic antibodies (ANCA) against bactericidal/permeability-increasing protein (BPI) and cystic fibrosis lung disease.Clin Exp Immunol. 1999 Sep;117(3):561-7. doi: 10.1046/j.1365-2249.1999.01006.x. Clin Exp Immunol. 1999. PMID: 10469063 Free PMC article.
-
Mammalian antibiotic peptides.Folia Microbiol (Praha). 2003;48(2):123-37. doi: 10.1007/BF02930945. Folia Microbiol (Praha). 2003. PMID: 12800493 Review.
-
L-4F inhibits lipopolysaccharide-mediated activation of primary human neutrophils.Inflammation. 2014 Oct;37(5):1401-12. doi: 10.1007/s10753-014-9864-7. Inflammation. 2014. PMID: 24647607 Free PMC article.
-
BPI-ANCA and long-term prognosis among 46 adult CF patients: a prospective 10-year follow-up study.Clin Dev Immunol. 2012;2012:370107. doi: 10.1155/2012/370107. Epub 2012 Dec 30. Clin Dev Immunol. 2012. PMID: 23346184 Free PMC article.
-
Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages.Cell Microbiol. 2005 Sep;7(9):1251-62. doi: 10.1111/j.1462-5822.2005.00549.x. Cell Microbiol. 2005. PMID: 16098213 Free PMC article.
References
-
- Schumann R R, Leong S R, Flaggs G W, Gray P W, Wright S D, Mathison J C, Tobias P S, Ulevitch R J. Science. 1990;249:1429–1431. - PubMed
-
- Ulevitch R J, Tobias P S. Annu Rev Immunol. 1995;13:437–457. - PubMed
-
- Elsbach P, Weiss J. Immunobiology. 1993;187:417–429. - PubMed
-
- Zarember K, Elsbach P, Kim K-S, Weiss J. Blood. 1997;89:672–679. - PubMed
-
- Han J, Mathison J C, Ulevitch R J, Tobias P S. J Biol Chem. 1994;269:8172–8175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

