Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells
- PMID: 9380693
- PMCID: PMC23442
- DOI: 10.1073/pnas.94.20.10669
Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells
Abstract
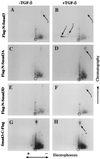
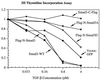
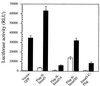
Drosophila Mad proteins are intracellular signal transducers of decapentaplegic (dpp), the Drosophila transforming growth factor beta (TGF-beta)/bone morphogenic protein (BMP) homolog. Studies in which the mammalian Smad homologs were transiently overexpressed in cultured cells have implicated Smad2 in TGF-beta signaling, but the physiological relevance of the Smad3 protein in signaling by TGF-beta receptors has not been established. Here we stably expressed Smad proteins at controlled levels in epithelial cells using a novel approach that combines highly efficient retroviral gene transfer and quantitative cell sorting. We show that upon TGF-beta treatment Smad3 becomes rapidly phosphorylated at the SSVS motif at its very C terminus. Either attachment of an epitope tag to the C terminus or replacement of these three serine residues with alanine abolishes TGF-beta-induced Smad3 phosphorylation; these proteins act in a dominant-negative fashion to block the antiproliferative effect of TGF-beta in mink lung epithelial cells. A Smad3 protein in which the three C-terminal serines have been replaced by aspartic acids is also a dominant inhibitor of TGF-beta signaling, but can activate plasminogen activator inhibitor 1 (PAI-1) transcription in a ligand-independent fashion when its nuclear localization is forced by transient overexpression. Phosphorylation of the three C-terminal serine residues of Smad3 by an activated TGF-beta receptor complex is an essential step in signal transduction by TGF-beta for both inhibition of cell proliferation and activation of the PAI-1 promoter.
Figures


Similar articles
-
TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4.EMBO J. 1997 Sep 1;16(17):5353-62. doi: 10.1093/emboj/16.17.5353. EMBO J. 1997. PMID: 9311995 Free PMC article.
-
Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-beta signaling.J Biol Chem. 1997 Oct 31;272(44):28107-15. doi: 10.1074/jbc.272.44.28107. J Biol Chem. 1997. PMID: 9346966
-
The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells.Kidney Int. 1999 Oct;56(4):1354-65. doi: 10.1046/j.1523-1755.1999.00680.x. Kidney Int. 1999. PMID: 10504488
-
Transforming growth factor-beta and breast cancer: Transforming growth factor-beta/SMAD signaling defects and cancer.Breast Cancer Res. 2000;2(2):107-15. doi: 10.1186/bcr42. Epub 2000 Feb 21. Breast Cancer Res. 2000. PMID: 11250700 Free PMC article. Review.
-
Phosphorylation status at Smad3 linker region modulates transforming growth factor-β-induced epithelial-mesenchymal transition and cancer progression.Cancer Sci. 2019 Feb;110(2):481-488. doi: 10.1111/cas.13922. Epub 2019 Jan 23. Cancer Sci. 2019. PMID: 30589983 Free PMC article. Review.
Cited by
-
Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells.Autophagy. 2013 May;9(5):730-42. doi: 10.4161/auto.24039. Epub 2013 Mar 20. Autophagy. 2013. PMID: 23514933 Free PMC article.
-
Hypoxia-inducible factor-1α promotes glomerulosclerosis and regulates COL1A2 expression through interactions with Smad3.Kidney Int. 2016 Oct;90(4):797-808. doi: 10.1016/j.kint.2016.05.026. Epub 2016 Aug 5. Kidney Int. 2016. PMID: 27503806 Free PMC article.
-
Blood components for topical use in tissue regeneration: evaluation of corneal lesions treated with platelet lysate and considerations on repair mechanisms.Blood Transfus. 2010 Apr;8(2):107-12. doi: 10.2450/2009.0091-09. Blood Transfus. 2010. PMID: 20383304 Free PMC article.
-
Post-transcriptional up-regulation of Tsc-22 by Ybx1, a target of miR-216a, mediates TGF-{beta}-induced collagen expression in kidney cells.J Biol Chem. 2010 Oct 29;285(44):34004-15. doi: 10.1074/jbc.M110.165027. Epub 2010 Aug 16. J Biol Chem. 2010. PMID: 20713358 Free PMC article.
-
Identification and characterization of regulator of G protein signaling 4 (RGS4) as a novel inhibitor of tubulogenesis: RGS4 inhibits mitogen-activated protein kinases and vascular endothelial growth factor signaling.Mol Biol Cell. 2005 Feb;16(2):609-25. doi: 10.1091/mbc.e04-06-0479. Epub 2004 Nov 17. Mol Biol Cell. 2005. PMID: 15548600 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

