KSR stimulates Raf-1 activity in a kinase-independent manner
- PMID: 9371754
- PMCID: PMC24217
- DOI: 10.1073/pnas.94.24.12792
KSR stimulates Raf-1 activity in a kinase-independent manner
Erratum in
- Proc Natl Acad Sci U S A 1998 mAR 3;95(5):2714-5
Abstract
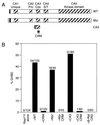
Kinase suppressor of Ras (KSR) is an evolutionarily conserved component of Ras-dependent signaling pathways. Here, we find that murine KSR (mKSR1) translocates from the cytoplasm to the plasma membrane in the presence of activated Ras. At the membrane, mKSR1 modulates Ras signaling by enhancing Raf-1 activity in a kinase-independent manner. The activation of Raf-1 is mediated by the mKSR1 cysteine-rich CA3 domain and involves a detergent labile cofactor that is not ceramide. These findings reveal another point of regulation for Ras-mediated signal transduction and further define a noncatalytic role for mKSR1 in the multistep process of Raf-1 activation.
Figures
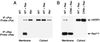


Similar articles
-
KSR modulates signal propagation within the MAPK cascade.Genes Dev. 1996 Nov 1;10(21):2684-95. doi: 10.1101/gad.10.21.2684. Genes Dev. 1996. PMID: 8946910
-
Solution structure and functional analysis of the cysteine-rich C1 domain of kinase suppressor of Ras (KSR).J Mol Biol. 2002 Jan 18;315(3):435-46. doi: 10.1006/jmbi.2001.5263. J Mol Biol. 2002. PMID: 11786023
-
Kinase suppressor of ras is necessary for tumor necrosis factor alpha activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells.Cancer Res. 2001 Feb 1;61(3):963-9. Cancer Res. 2001. PMID: 11221891
-
Untying the regulation of the Raf-1 kinase.Arch Biochem Biophys. 2002 Aug 1;404(1):3-9. doi: 10.1016/s0003-9861(02)00244-8. Arch Biochem Biophys. 2002. PMID: 12127063 Review.
-
Ras signaling: PP2A puts Ksr and Raf in the right place.Curr Biol. 2003 Aug 19;13(16):R635-7. doi: 10.1016/s0960-9822(03)00568-2. Curr Biol. 2003. PMID: 12932339 Review. No abstract available.
Cited by
-
Tumor biomarkers for diagnosis, prognosis and targeted therapy.Signal Transduct Target Ther. 2024 May 20;9(1):132. doi: 10.1038/s41392-024-01823-2. Signal Transduct Target Ther. 2024. PMID: 38763973 Free PMC article. Review.
-
Targeting CRAF kinase in anti-cancer therapy: progress and opportunities.Mol Cancer. 2023 Dec 18;22(1):208. doi: 10.1186/s12943-023-01903-x. Mol Cancer. 2023. PMID: 38111008 Free PMC article. Review.
-
Navigating the ERK1/2 MAPK Cascade.Biomolecules. 2023 Oct 20;13(10):1555. doi: 10.3390/biom13101555. Biomolecules. 2023. PMID: 37892237 Free PMC article. Review.
-
Ras Mitogen-activated Protein Kinase Signaling and Kinase Suppressor of Ras as Therapeutic Targets for Hepatocellular Carcinoma.J Liver Cancer. 2021 Mar;21(1):1-11. doi: 10.17998/jlc.21.1.1. Epub 2021 Mar 31. J Liver Cancer. 2021. PMID: 37384270 Free PMC article. Review.
-
Pseudokinase NRP1 facilitates endocytosis of transferrin in the African trypanosome.Sci Rep. 2022 Nov 3;12(1):18572. doi: 10.1038/s41598-022-22054-x. Sci Rep. 2022. PMID: 36329148 Free PMC article.
References
-
- Therrien M, Michaud N R, Rubin G M, Morrison D K. Genes Dev. 1996;10:2684–2695. - PubMed
-
- Therrien M, Chang H C, Solomon N M, Karim F D, Wassarman D A, Rubin G M. Cell. 1995;83:879–888. - PubMed
-
- Sundaram M, Han M. Cell. 1995;83:889–901. - PubMed
-
- Kornfeld K, Hom D B, Horvitz H R. Cell. 1995;83:903–913. - PubMed
-
- Xing H, Kornfeld K, Muslin A J. Curr Biol. 1997;7:294–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

