A sponge-like structure involved in the association and transport of maternal products during Drosophila oogenesis
- PMID: 9348297
- PMCID: PMC2141720
- DOI: 10.1083/jcb.139.3.817
A sponge-like structure involved in the association and transport of maternal products during Drosophila oogenesis
Abstract
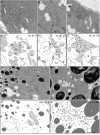
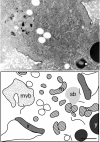
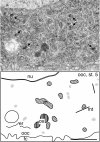
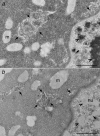
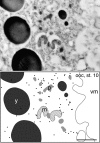
Localization of maternally provided RNAs during oogenesis is required for formation of the antero-posterior axis of the Drosophila embryo. Here we describe a subcellular structure in nurse cells and oocytes which may function as an intracellular compartment for assembly and transport of maternal products involved in RNA localization. This structure, which we have termed "sponge body," consists of ER-like cisternae, embedded in an amorphous electron-dense mass. It lacks a surrounding membrane and is frequently associated with mitochondria. The sponge bodies are not identical to the Golgi complexes. We suggest that the sponge bodies are homologous to the mitochondrial cloud in Xenopus oocytes, a granulo-fibrillar structure that contains RNAs involved in patterning of the embryo. Exuperantia protein, the earliest factor known to be required for the localization of bicoid mRNA to the anterior pole of the Drosophila oocyte, is highly enriched in the sponge bodies but not an essential structural component of these. RNA staining indicates that sponge bodies contain RNA. However, neither the intensity of this staining nor the accumulation of Exuperantia in the sponge bodies is dependent on the amount of bicoid mRNA present in the ovaries. Sponge bodies surround nuage, a possible polar granule precursor. Microtubules and microfilaments are not present in sponge bodies, although transport of the sponge bodies through the cells is implied by their presence in cytoplasmic bridges. We propose that the sponge bodies are structures that, by assembly and transport of included molecules or associated structures, are involved in localization of mRNAs in Drosophila oocytes.
Figures








Similar articles
-
In vivo analyses of cytoplasmic transport and cytoskeletal organization during Drosophila oogenesis: characterization of a multi-step anterior localization pathway.Development. 1998 Sep;125(18):3655-66. doi: 10.1242/dev.125.18.3655. Development. 1998. PMID: 9716531
-
The temporal and spatial distribution pattern of maternal exuperantia protein: evidence for a role in establishment but not maintenance of bicoid mRNA localization.EMBO J. 1991 Dec;10(13):4259-66. doi: 10.1002/j.1460-2075.1991.tb05004.x. EMBO J. 1991. PMID: 1756733 Free PMC article.
-
The molecular motor dynein is involved in targeting swallow and bicoid RNA to the anterior pole of Drosophila oocytes.Nat Cell Biol. 2000 Apr;2(4):185-90. doi: 10.1038/35008601. Nat Cell Biol. 2000. PMID: 10783235
-
Microtubule polarity and axis formation in the Drosophila oocyte.Dev Dyn. 2006 Jun;235(6):1455-68. doi: 10.1002/dvdy.20770. Dev Dyn. 2006. PMID: 16586443 Review.
-
Axis formation during Drosophila oogenesis.Curr Opin Genet Dev. 2001 Aug;11(4):374-83. doi: 10.1016/s0959-437x(00)00207-0. Curr Opin Genet Dev. 2001. PMID: 11448623 Review.
Cited by
-
Intercellular organelle traffic through cytoplasmic bridges in early spermatids of the rat: mechanisms of haploid gene product sharing.Mol Biol Cell. 2003 Jul;14(7):2768-80. doi: 10.1091/mbc.e02-10-0647. Epub 2003 Apr 4. Mol Biol Cell. 2003. PMID: 12857863 Free PMC article.
-
The Yb protein defines a novel organelle and regulates male germline stem cell self-renewal in Drosophila melanogaster.J Cell Biol. 2009 May 18;185(4):613-27. doi: 10.1083/jcb.200903034. Epub 2009 May 11. J Cell Biol. 2009. PMID: 19433453 Free PMC article.
-
Drosophila patterning is established by differential association of mRNAs with P bodies.Nat Cell Biol. 2012 Dec;14(12):1305-13. doi: 10.1038/ncb2627. Epub 2012 Nov 25. Nat Cell Biol. 2012. PMID: 23178881 Free PMC article.
-
Isolation of a ribonucleoprotein complex involved in mRNA localization in Drosophila oocytes.J Cell Biol. 2000 Feb 7;148(3):427-40. doi: 10.1083/jcb.148.3.427. J Cell Biol. 2000. PMID: 10662770 Free PMC article.
-
Bicaudal C and trailer hitch have similar roles in gurken mRNA localization and cytoskeletal organization.Dev Biol. 2009 Apr 15;328(2):434-44. doi: 10.1016/j.ydbio.2009.02.003. Epub 2009 Feb 13. Dev Biol. 2009. PMID: 19217894 Free PMC article.
References
-
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969;27:250–265. - PubMed
-
- Bohrmann J, Biber K. Cytoskeleton-dependent transport of cytoplasmic particles in previtellogenic to mid-vitellogenic ovarian follicles of Drosophila: time-lapse analysis using video-enhanced contrast microscopy. J Cell Sci. 1994;107:849–858. - PubMed
-
- Breitwieser W, Markussen F-H, Horstmann H, Ephrussi A. Oskar protein interaction with Vasa represents an essential step in polar granule assembly. Genes Dev. 1996;10:2179–2188. - PubMed
-
- Challacombe JF, Snow DM, Letourneau PC. Actin filament bundles are required for microtubule reorientation during growth cone turning to avoid an inhibitory guidance cue. J Cell Sci. 1996;109:2031–2040. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

