Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments
- PMID: 9348281
- PMCID: PMC2141717
- DOI: 10.1083/jcb.139.3.639
Major histocompatibility complex class II compartments in human and mouse B lymphoblasts represent conventional endocytic compartments
Abstract
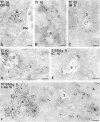
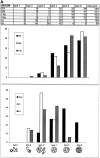
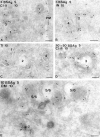
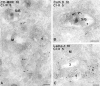
In most human and mouse antigen-presenting cells, the majority of intracellular major histocompatibility complex (MHC) class II molecules resides in late endocytic MHC class II compartments (MIICs), thought to function in antigen processing and peptide loading. However, in mouse A20 B cells, early endocytic class II-containing vesicles (CIIVs) have been reported to contain most of the intracellular MHC class II molecules and have also been implicated in formation of MHC class II-peptide complexes. To address this discrepancy, we have studied in great detail the endocytic pathways of both a human (6H5.DM) and a mouse (A20.Ab) B cell line. Using quantitative immunoelectron microscopy on cryosections of cells that had been pulse-chased with transferrin-HRP or BSA-gold as endocytic tracers, we have identified up to six endocytic subcompartments including an early MIIC type enriched in invariant chain, suggesting that it serves as an important entrance to the endocytic pathway for newly synthesized MHC class II/invariant chain complexes. In addition, early MIICs represented the earliest endocytic compartment containing MHC class II- peptide complexes, as shown by using an antibody against an abundant endogenous class II-peptide complex. The early MIIC exhibited several though not all of the characteristics reported for the CIIV and was situated just downstream of early endosomes. We have not encountered any special class II-containing endocytic structures besides those normally present in nonantigen-presenting cells. Our results therefore suggest that B cells use conventional endocytic compartments rather than having developed a unique compartment to accomplish MHC class II presentation.
Figures



Similar articles
-
Major histocompatibility complex class II compartments in human B lymphoblastoid cells are distinct from early endosomes.J Exp Med. 1995 Aug 1;182(2):325-34. doi: 10.1084/jem.182.2.325. J Exp Med. 1995. PMID: 7629497 Free PMC article.
-
HLA-DM is localized to conventional and unconventional MHC class II-containing endocytic compartments.Immunity. 1996 Mar;4(3):229-39. doi: 10.1016/s1074-7613(00)80431-8. Immunity. 1996. PMID: 8624813
-
Trafficking of major histocompatibility complex class II molecules through intracellular compartments containing HLA-DM.Hum Immunol. 1996 Jan;45(1):13-23. doi: 10.1016/0198-8859(95)00152-2. Hum Immunol. 1996. PMID: 8655355
-
MHC class II molecules on the move for successful antigen presentation.EMBO J. 2008 Jan 9;27(1):1-5. doi: 10.1038/sj.emboj.7601945. Epub 2007 Nov 29. EMBO J. 2008. PMID: 18046453 Free PMC article. Review.
-
Intracellular organelles involved in antigen processing and the binding of peptides to class II MHC molecules.Semin Immunol. 1995 Dec;7(6):355-60. doi: 10.1006/smim.1995.0040. Semin Immunol. 1995. PMID: 8775461 Review.
Cited by
-
The HSP90 inhibitor geldanamycin perturbs endosomal structure and drives recycling ErbB2 and transferrin to modified MVBs/lysosomal compartments.Mol Biol Cell. 2013 Jan;24(2):129-44. doi: 10.1091/mbc.E12-04-0282. Epub 2012 Nov 14. Mol Biol Cell. 2013. PMID: 23154999 Free PMC article.
-
Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function.Nat Rev Mol Cell Biol. 2009 Sep;10(9):623-35. doi: 10.1038/nrm2745. Epub 2009 Aug 12. Nat Rev Mol Cell Biol. 2009. PMID: 19672277 Review.
-
Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells.Mol Biol Cell. 1999 Dec;10(12):4163-76. doi: 10.1091/mbc.10.12.4163. Mol Biol Cell. 1999. PMID: 10588650 Free PMC article.
-
Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT).Proc Natl Acad Sci U S A. 2000 Jan 18;97(2):745-50. doi: 10.1073/pnas.97.2.745. Proc Natl Acad Sci U S A. 2000. PMID: 10639150 Free PMC article.
-
SARS coronavirus nucleocapsid immunodominant T-cell epitope cluster is common to both exogenous recombinant and endogenous DNA-encoded immunogens.Virology. 2006 Mar 30;347(1):127-39. doi: 10.1016/j.virol.2005.11.042. Epub 2006 Jan 4. Virology. 2006. PMID: 16387339 Free PMC article.
References
-
- Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature (Lond) 1994;369:113–120. - PubMed
-
- Bhattacharya A, Dorf ME, Springer TA. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981;127:2488–2495. - PubMed
-
- Bikoff EK, Germain RN, Robertson EJ. Allelic differences affecting invariant chain dependency of MHC class II subunit assembly. Immunity. 1995;2:301–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

