Interleukin 3-dependent survival by the Akt protein kinase
- PMID: 9326612
- PMCID: PMC23462
- DOI: 10.1073/pnas.94.21.11345
Interleukin 3-dependent survival by the Akt protein kinase
Abstract
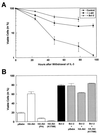
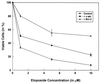
Interleukin 3 (IL-3)-dependent survival of hematopoietic cells is known to rely on the activity of multiple signaling pathways, including a pathway leading to activation of phosphoinositide 3-kinase (PI 3-kinase), and protein kinase Akt is a direct target of PI 3-kinase. We find that Akt kinase activity is rapidly induced by the cytokine IL-3, suggesting a role for Akt in PI 3-kinase-dependent signaling in hematopoetic cells. Dominant-negative mutants of Akt specifically block Akt activation by IL-3 and interfere with IL-3-dependent proliferation. Overexpression of Akt or oncogenic v-akt protects 32D cells from apoptosis induced by IL-3 withdrawal. Apoptosis after IL-3 withdrawal is accelerated by expression of dominant-negative mutants of Akt, indicating that a functional Akt signaling pathway is necessary for cell survival mediated by the cytokine IL-3. Thus Akt appears to be an important mediator of anti-apoptotic signaling in this system.
Figures



Similar articles
-
TEL-Syk fusion constitutively activates PI3-K/Akt, MAPK and JAK2-independent STAT5 signal pathways.Leukemia. 2004 Mar;18(3):548-55. doi: 10.1038/sj.leu.2403266. Leukemia. 2004. PMID: 14749700
-
Requirement for the PI3K/Akt pathway in MEK1-mediated growth and prevention of apoptosis: identification of an Achilles heel in leukemia.Leukemia. 2003 Jun;17(6):1058-67. doi: 10.1038/sj.leu.2402925. Leukemia. 2003. PMID: 12764369
-
Regulation and function of protein kinase B and MAP kinase activation by the IL-5/GM-CSF/IL-3 receptor.Oncogene. 1999 Jun 3;18(22):3334-42. doi: 10.1038/sj.onc.1202678. Oncogene. 1999. PMID: 10362354
-
Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival.Cell Cycle. 2003 May-Jun;2(3):220-3. Cell Cycle. 2003. PMID: 12734428 Review.
-
Regulation of survival pathways by IL-3 and induction of apoptosis following IL-3 withdrawal.Front Biosci. 1998 Mar 15;3:d313-24. doi: 10.2741/a276. Front Biosci. 1998. PMID: 9488657 Review.
Cited by
-
Canine distemper virus N protein induces autophagy to facilitate viral replication.BMC Vet Res. 2023 Mar 15;19(1):60. doi: 10.1186/s12917-023-03575-7. BMC Vet Res. 2023. PMID: 36922800 Free PMC article.
-
Multi-objective optimization reveals time- and dose-dependent inflammatory cytokine-mediated regulation of human stem cell derived T-cell development.NPJ Regen Med. 2022 Jan 27;7(1):11. doi: 10.1038/s41536-022-00210-1. NPJ Regen Med. 2022. PMID: 35087040 Free PMC article.
-
Cellular model system to dissect the isoform-selectivity of Akt inhibitors.Nat Commun. 2021 Sep 6;12(1):5297. doi: 10.1038/s41467-021-25512-8. Nat Commun. 2021. PMID: 34489430 Free PMC article.
-
Natural Antioxidant and Anti-Inflammatory Compounds in Foodstuff or Medicinal Herbs Inducing Heme Oxygenase-1 Expression.Antioxidants (Basel). 2020 Nov 27;9(12):1191. doi: 10.3390/antiox9121191. Antioxidants (Basel). 2020. PMID: 33260980 Free PMC article. Review.
-
Boar seminal plasma: current insights on its potential role for assisted reproductive technologies in swine.Anim Reprod. 2020 Jul 21;17(3):e20200022. doi: 10.1590/1984-3143-AR2020-0022. Anim Reprod. 2020. PMID: 33029213 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

