The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos
- PMID: 9265652
- PMCID: PMC2138053
- DOI: 10.1083/jcb.138.4.861
The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos
Abstract
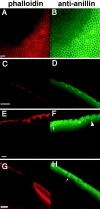
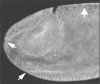
Syntaxins are membrane proteins involved in vesicle trafficking and are required for the release of neurotransmitter at nerve terminals. The presence of syntaxins on target membranes has been hypothesized to confer specificity to targeting and fusion via interactions with complementary vesicle-associated proteins, the synaptobrevins or VAMPS. We have mutagenized syntaxin1 in Drosophila and have found that it links the mechanism of synaptic transmission to a distinct cell biological process: the cellularization of early embryos. This specialized form of cell division separates the 6,000 nuclei of the syncytial blastoderm into separate cells through the invagination of the surface membrane of the embryo. During this process, syntaxin1 protein is present on the newly forming lateral cell surfaces and invaginating cleavage furrows. This protein is derived both from maternal deposition of mRNA and protein and from early zygotic transcription. To analyze syntaxin1's role in early development, female germ line mosaics mutant for syntaxin1 expression were generated by mitotic recombination to reduce the maternal contribution. Visualizing the actin cytoskeleton and glycosylated surface proteins reveals that embryos with insufficient syntaxin1 have large acellular patches. The patches do not appear until cellularization begins, and the process fails entirely within these regions. These results provide genetic evidence that membrane trafficking is required for the cellularization of the syncytial blastoderm. We propose that the invagination of the surface membrane proceeds by the fusion of intracellular membrane vesicles with the surface. This reaction uses the same syntaxin1 protein as is required for neurotransmitter secretion at synapses. Thus, a single syntaxin can participate in trafficking steps that are functionally as distinct as synaptic transmission and cell division.
Figures





Similar articles
-
Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization.Mol Biol Cell. 2000 Sep;11(9):3123-35. doi: 10.1091/mbc.11.9.3123. Mol Biol Cell. 2000. PMID: 10982405 Free PMC article.
-
Cellularization in Drosophila melanogaster is disrupted by the inhibition of rho activity and the activation of Cdc42 function.Dev Biol. 1998 Dec 1;204(1):151-64. doi: 10.1006/dbio.1998.9061. Dev Biol. 1998. PMID: 9851849
-
The serendipity alpha gene encodes a membrane-associated protein required for the cellularization of the Drosophila embryo.Genes Dev. 1990 Jun;4(6):922-31. doi: 10.1101/gad.4.6.922. Genes Dev. 1990. PMID: 2166703
-
TRP Channel Trafficking.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
-
How one becomes many: blastoderm cellularization in Drosophila melanogaster.Bioessays. 2002 Nov;24(11):1012-22. doi: 10.1002/bies.10184. Bioessays. 2002. PMID: 12386932 Review.
Cited by
-
A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype.Genetics. 1999 Aug;152(4):1631-9. doi: 10.1093/genetics/152.4.1631. Genetics. 1999. PMID: 10430588 Free PMC article.
-
Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization.J Cell Biol. 2000 Nov 13;151(4):905-18. doi: 10.1083/jcb.151.4.905. J Cell Biol. 2000. PMID: 11076973 Free PMC article.
-
Genetic dissection of cytokinesis.Plant Mol Biol. 2000 Aug;43(5-6):719-33. doi: 10.1023/a:1006457723760. Plant Mol Biol. 2000. PMID: 11089872 Review.
-
The plasma membrane flattens out to fuel cell-surface growth during Drosophila cellularization.Dev Cell. 2013 Dec 23;27(6):648-55. doi: 10.1016/j.devcel.2013.11.006. Epub 2013 Dec 5. Dev Cell. 2013. PMID: 24316147 Free PMC article.
-
Ultrastructure and Membrane Traffic During Cell Division in the Marine Pennate Diatom Phaeodactylum tricornutum.Protist. 2015 Nov;166(5):506-21. doi: 10.1016/j.protis.2015.07.005. Epub 2015 Aug 14. Protist. 2015. PMID: 26386358 Free PMC article.
References
-
- Ashburner, M. 1989. Drosophila, A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 434 pp.
-
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science (Wash DC) 1992;257:255–259. - PubMed
-
- Bier E, Vaessin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto R, Uemura T, Grell E, et al. Searching for pattern and mutation in the Drosophila genome with a P-lacZvector. Genes Dev. 1989;3:1273–1287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

