Mutagenesis reveals structure-activity parallels between human A2A adenosine receptors and biogenic amine G protein-coupled receptors
- PMID: 9258366
- PMCID: PMC3449164
- DOI: 10.1021/jm970084v
Mutagenesis reveals structure-activity parallels between human A2A adenosine receptors and biogenic amine G protein-coupled receptors
Abstract
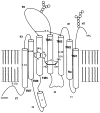
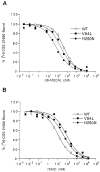
Structure-affinity relationships for ligand binding at the human A2A adenosine receptor have been probed using site-directed mutagenesis in the transmembrane helical domains (TMs). The mutant receptors were expressed in COS-7 cells and characterized by binding of the radioligands [3H]CGS21680, [3H]NECA, and [3H]XAC. Three residues, at positions essential for ligand binding in other G protein-coupled receptors, were individually mutated. The residue V(3.32) in the A2A receptor that is homologous to the essential aspartate residue of TM3 in the biogenic amine receptors, i.e., V84(3.32), may be substituted with L (present in the A3 receptor) but not with D (in biogenic amine receptors) or A. H250(6.52), homologous to the critical N507 of rat m3 muscarinic acetylcholine receptors, may be substituted with other aromatic residues or with N but not with A (Kim et al. J. Biol. Chem. 1995, 270, 13987-13997). H278(7.43), homologous to the covalent ligand anchor site in rhodopsin, may not be substituted with either A, K, or N. Both V84L(3.32) and H250N(6.52) mutant receptors were highly variable in their effect on ligand competition depending on the structural class of the ligand. Adenosine-5'-uronamide derivatives were more potent at the H250N(6.52) mutant receptor than at wild type receptors. Xanthines tended to be close in potency (H250N(6.52)) or less potent (V84L(3.32)) than at wild type receptors. The affinity of CGS21680 increased as the pH was lowered to 5.5 in both the wild type and H250N(6.52) mutant receptors. Thus, protonation of H250(6.52) is not involved in this pH dependence. These data are consistent with a molecular model predicting the proximity of bound agonist ligands to TM3, TM5, TM6, and TM7.
Figures

Similar articles
-
Glutamate residues in the second extracellular loop of the human A2a adenosine receptor are required for ligand recognition.Mol Pharmacol. 1996 Apr;49(4):683-91. Mol Pharmacol. 1996. PMID: 8609897 Free PMC article.
-
A carboxyl-terminally truncated mutant and nonglycosylated A2a adenosine receptors retain ligand binding.Mol Pharmacol. 1994 May;45(5):861-70. Mol Pharmacol. 1994. PMID: 8190103
-
Characterization of a low affinity binding site for N6-substituted adenosine derivatives in rat testis membranes.J Recept Signal Transduct Res. 1995 Sep-Dec;15(7-8):905-29. doi: 10.3109/10799899509049864. J Recept Signal Transduct Res. 1995. PMID: 8673723
-
Site-directed mutagenesis identifies residues involved in ligand recognition in the human A2a adenosine receptor.J Biol Chem. 1995 Jun 9;270(23):13987-97. doi: 10.1074/jbc.270.23.13987. J Biol Chem. 1995. PMID: 7775460 Free PMC article.
-
Identification of domains of the human A1 adenosine receptor that are important for binding receptor subtype-selective ligands using chimeric A1/A2a adenosine receptors.J Biol Chem. 1995 Sep 1;270(35):20485-90. doi: 10.1074/jbc.270.35.20485. J Biol Chem. 1995. PMID: 7657625
Cited by
-
Structural probing of off-target G protein-coupled receptor activities within a series of adenosine/adenine congeners.PLoS One. 2014 May 23;9(5):e97858. doi: 10.1371/journal.pone.0097858. eCollection 2014. PLoS One. 2014. PMID: 24859150 Free PMC article.
-
Structure-function of the G protein-coupled receptor superfamily.Annu Rev Pharmacol Toxicol. 2013;53:531-56. doi: 10.1146/annurev-pharmtox-032112-135923. Epub 2012 Nov 8. Annu Rev Pharmacol Toxicol. 2013. PMID: 23140243 Free PMC article. Review.
-
A neoceptor approach to unraveling microscopic interactions between the human A2A adenosine receptor and its agonists.Chem Biol. 2005 Feb;12(2):237-47. doi: 10.1016/j.chembiol.2004.12.010. Chem Biol. 2005. PMID: 15734651 Free PMC article.
-
Ribose modified nucleosides and nucleotides as ligands for purine receptors.Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):333-41. doi: 10.1081/NCN-100002305. Nucleosides Nucleotides Nucleic Acids. 2001. PMID: 11563046 Free PMC article. Review.
-
Arginine 199 and leucine 208 have key roles in the control of adenosine A2A receptor signalling function.PLoS One. 2014 Mar 3;9(3):e89613. doi: 10.1371/journal.pone.0089613. eCollection 2014. PLoS One. 2014. PMID: 24595172 Free PMC article.
References
-
- Libert F, Parmentier M, Lefort A, Dinsart C, van Sande J, Maenhaut C, Simons MJ, Dumont JE, Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244:569–572. - PubMed
-
- Jacobson M. Molecular biology of adenosine receptors. In: Belardinelli L, Pelleg A, editors. Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology. Kluver; Norwell, MA: 1995. pp. 5–14.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

