P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase
- PMID: 9256433
- PMCID: PMC23024
- DOI: 10.1073/pnas.94.17.9052
P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase
Abstract
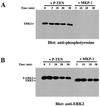
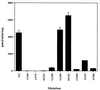
Protein tyrosine phosphatases (PTPs) have long been thought to play a role in tumor suppression due to their ability to antagonize the growth promoting protein tyrosine kinases. Recently, a candidate tumor suppressor from 10q23, termed P-TEN, was isolated, and sequence homology was demonstrated with members of the PTP family, as well as the cytoskeletal protein tensin. Here we show that recombinant P-TEN dephosphorylated protein and peptide substrates phosphorylated on serine, threonine, and tyrosine residues, indicating that P-TEN is a dual-specificity phosphatase. In addition, P-TEN exhibited a high degree of substrate specificity, showing selectivity for extremely acidic substrates in vitro. Furthermore, we demonstrate that mutations in P-TEN, identified from primary tumors, tumor cells lines, and a patient with Bannayan-Zonana syndrome, resulted in the ablation of phosphatase activity, demonstrating that enzymatic activity of P-TEN is necessary for its ability to function as a tumor suppressor.
Figures


Similar articles
-
Germline mutations in PTEN are present in Bannayan-Zonana syndrome.Nat Genet. 1997 Aug;16(4):333-4. doi: 10.1038/ng0897-333. Nat Genet. 1997. PMID: 9241266 No abstract available.
-
Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation.Hum Mol Genet. 1998 Mar;7(3):507-15. doi: 10.1093/hmg/7.3.507. Hum Mol Genet. 1998. PMID: 9467011
-
PTEN and myotubularin: novel phosphoinositide phosphatases.Annu Rev Biochem. 2001;70:247-79. doi: 10.1146/annurev.biochem.70.1.247. Annu Rev Biochem. 2001. PMID: 11395408 Review.
-
TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta.Cancer Res. 1997 Jun 1;57(11):2124-9. Cancer Res. 1997. PMID: 9187108
-
Phosphatases and tumorigenesis.Curr Opin Oncol. 1998 Jan;10(1):88-91. doi: 10.1097/00001622-199801000-00014. Curr Opin Oncol. 1998. PMID: 9466490 Review.
Cited by
-
A novel human TPIP splice-variant (TPIP-C2) mRNA, expressed in human and mouse tissues, strongly inhibits cell growth in HeLa cells.PLoS One. 2011;6(12):e28433. doi: 10.1371/journal.pone.0028433. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164291 Free PMC article.
-
The HECT family of E3 ubiquitin ligases and PTEN.Semin Cancer Biol. 2022 Oct;85:43-51. doi: 10.1016/j.semcancer.2021.06.012. Epub 2021 Jun 12. Semin Cancer Biol. 2022. PMID: 34129913 Free PMC article. Review.
-
Saturated Fatty Acid-induced cytotoxicity in liver cells does not involve phosphatase and tensin homologue deleted on chromosome 10.J Nutr Metab. 2013;2013:514206. doi: 10.1155/2013/514206. Epub 2013 Apr 15. J Nutr Metab. 2013. PMID: 23691291 Free PMC article.
-
Papillary carcinoma occurring within an adenomatous goiter of the thyroid gland in Cowden's disease.Endocr Pathol. 2001 Spring;12(1):73-6. doi: 10.1385/ep:12:1:73. Endocr Pathol. 2001. PMID: 11478271
-
MicroRNA-21-5p acts via the PTEN/Akt/FOXO3a signaling pathway to prevent cardiomyocyte injury caused by high glucose/high fat conditions.Exp Ther Med. 2022 Mar;23(3):230. doi: 10.3892/etm.2022.11154. Epub 2022 Jan 18. Exp Ther Med. 2022. PMID: 35222707 Free PMC article.
References
-
- Rosen N. In: Molecular Basis of Cancer. Mendelsohn V, Howley P M, Israel M A, Liota L A, editors. Philadelphia: Saunders; 1995. pp. 105–140.
-
- Gauwerky C E, Croce C M. In: Molecular Basis of Cancer. Mendelsohn V, Howley P M, Israel M A, Liota L A, editors. Philadelphia: Saunders; 1995. pp. 18–37.
-
- Steck P A, Perhouse M A, Jasser S A, Yung W K A, Lin H, Ligon A H, Lauren A L, Baumgard M L, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng D H F, Tavtigan S V. Nat Genet. 1997;15:356–362. - PubMed
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S, Puc J, Milliaresis C, Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittman M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. Science. 1997;275:1943–1946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

