Novel beta-secretase cleavage of beta-amyloid precursor protein in the endoplasmic reticulum/intermediate compartment of NT2N cells
- PMID: 9245794
- PMCID: PMC2141643
- DOI: 10.1083/jcb.138.3.671
Novel beta-secretase cleavage of beta-amyloid precursor protein in the endoplasmic reticulum/intermediate compartment of NT2N cells
Abstract
Previous studies have demonstrated that NT2N neurons derived from a human embryonal carcinoma cell line (NT2) constitutively process the endogenous wild-type beta-amyloid precursor protein (APP) to amyloid beta peptide in an intracellular compartment. These studies indicate that other proteolytic fragments generated by intracellular processing must also be present in these cells. Here we show that the NH2-terminal fragment of APP generated by beta-secretase cleavage (APPbeta) is indeed produced from the endogenous full length APP (APPFL). Pulse-chase studies demonstrated a precursor-product relationship between APPFL and APPbeta as well as intracellular and secreted APPbeta fragments. In addition, trypsin digestion of intact NT2N cells at 4 degrees C did not abolish APPbeta recovered from the cell lysates. Furthermore, the production of intracellular APPbeta from wild-type APP appears to be a unique characteristic of postmitotic neurons, since intracellular APPbeta was not detected in several non-neuronal cell lines. Significantly, production of APPbeta occurred even when APP was retained in the ER/ intermediate compartment by inhibition with brefeldin A, incubation at 15 degrees C, or by expression of exogenous APP bearing the dilysine ER retrieval motif.
Figures
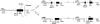






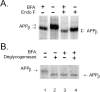


Similar articles
-
Novel alpha-secretase cleavage of Alzheimer's amyloid beta precursor protein in the endoplasmic reticulum of COS7 cells.Neurosci Lett. 2005 Mar 7;376(1):14-9. doi: 10.1016/j.neulet.2004.11.032. Epub 2004 Dec 8. Neurosci Lett. 2005. PMID: 15694266
-
The discrepancy between presenilin subcellular localization and gamma-secretase processing of amyloid precursor protein.J Cell Biol. 2001 Aug 20;154(4):731-40. doi: 10.1083/jcb.200104045. Epub 2001 Aug 13. J Cell Biol. 2001. PMID: 11502763 Free PMC article.
-
Cleavage of Alzheimer's amyloid precursor protein (APP) by secretases occurs after O-glycosylation of APP in the protein secretory pathway. Identification of intracellular compartments in which APP cleavage occurs without using toxic agents that interfere with protein metabolism.J Biol Chem. 1998 Mar 13;273(11):6277-84. doi: 10.1074/jbc.273.11.6277. J Biol Chem. 1998. PMID: 9497354
-
A distinct ER/IC gamma-secretase competes with the proteasome for cleavage of APP.Biochemistry. 2000 Feb 1;39(4):810-7. doi: 10.1021/bi991728z. Biochemistry. 2000. PMID: 10651647
-
Distinct presenilin-dependent and presenilin-independent gamma-secretases are responsible for total cellular Abeta production.J Neurosci Res. 2003 Nov 1;74(3):361-9. doi: 10.1002/jnr.10776. J Neurosci Res. 2003. PMID: 14598312 Review.
Cited by
-
Advances in the cell biology of the trafficking and processing of amyloid precursor protein: impact of familial Alzheimer's disease mutations.Biochem J. 2024 Oct 2;481(19):1297-1325. doi: 10.1042/BCJ20240056. Biochem J. 2024. PMID: 39302110 Free PMC article. Review.
-
Effects of TNFalpha-converting enzyme inhibition on amyloid beta production and APP processing in vitro and in vivo.J Neurosci. 2008 Nov 12;28(46):12052-61. doi: 10.1523/JNEUROSCI.2913-08.2008. J Neurosci. 2008. PMID: 19005070 Free PMC article.
-
Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons.J Cell Biol. 2002 Feb 4;156(3):519-29. doi: 10.1083/jcb.200110119. Epub 2002 Jan 28. J Cell Biol. 2002. PMID: 11815632 Free PMC article.
-
Use of yeast as a model system to investigate protein conformational diseases.Mol Biotechnol. 2005 Jun;30(2):171-80. doi: 10.1385/MB:30:2:171. Mol Biotechnol. 2005. PMID: 15920289 Review.
-
Detection of a novel intraneuronal pool of insoluble amyloid beta protein that accumulates with time in culture.J Cell Biol. 1998 May 18;141(4):1031-9. doi: 10.1083/jcb.141.4.1031. J Cell Biol. 1998. PMID: 9585420 Free PMC article.
References
-
- Arai H, Lee VM-Y, Messinger M, Greenberg BD, Lowery DE, Trojanowski JQ. Expression patterns of β-amyloid precursor protein (βAPP) in neural and nonneural human tissues from Alzheimer's disease and control subjects. Ann Neurol. 1991;30:686–693. - PubMed
-
- Cai X-D, Golde TE, Younkin SG. Release of excess amyloid β protein from a mutant amyloid β protein precursor. Science (Wash DC) 1993;259:514–516. - PubMed
-
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the β-amyloid precursor protein in Alzheimer's disease increases β-protein production. Nature (Lond) 1992;360:672–674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

