Physiological response to long-term peripheral and central leptin infusion in lean and obese mice
- PMID: 9238071
- PMCID: PMC23177
- DOI: 10.1073/pnas.94.16.8878
Physiological response to long-term peripheral and central leptin infusion in lean and obese mice
Abstract
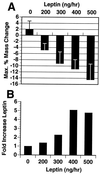
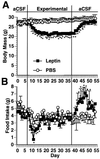
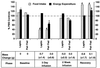
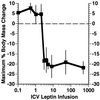
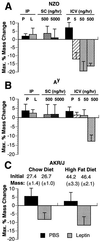
Recent data have identified leptin as an afferent signal in a negative-feedback loop regulating the mass of the adipose tissue. High leptin levels are observed in obese humans and rodents, suggesting that, in some cases, obesity is the result of leptin insensitivity. This hypothesis was tested by comparing the response to peripherally and centrally administered leptin among lean and three obese strains of mice: diet-induced obese AKR/J, New Zealand Obese (NZO), and Ay. Subcutaneous leptin infusion to lean mice resulted in a dose-dependent loss of body weight at physiologic plasma levels. Chronic infusions of leptin intracerebroventricularly (i.c.v.) at doses of 3 ng/hr or greater resulted in complete depletion of visible adipose tissue, which was maintained throughout 30 days of continuous i.c.v. infusion. Direct measurement of energy balance indicated that leptin treatment did not increase total energy expenditure but prevented the decrease that follows reduced food intake. Diet-induced obese mice lost weight in response to peripheral leptin but were less sensitive than lean mice. NZO mice were unresponsive to peripheral leptin but were responsive to i.c.v. leptin. Ay mice did not respond to subcutaneous leptin and were 1/100 as sensitive to i.c.v. leptin. The decreased response to leptin in diet-induced obese, NZO, and Ay mice suggests that obesity in these strains is the result of leptin resistance. In NZO mice, leptin resistance may be the result of decreased transport of leptin into the cerebrospinal fluid, whereas in Ay mice, leptin resistance probably results from defects downstream of the leptin receptor in the hypothalamus.
Figures
Similar articles
-
Hyperleptinemia, leptin resistance, and polymorphic leptin receptor in the New Zealand obese mouse.Endocrinology. 1997 Oct;138(10):4234-9. doi: 10.1210/endo.138.10.5428. Endocrinology. 1997. PMID: 9322935
-
Diet-induced obese mice develop peripheral, but not central, resistance to leptin.J Clin Invest. 1997 Feb 1;99(3):385-90. doi: 10.1172/JCI119171. J Clin Invest. 1997. PMID: 9022070 Free PMC article. Clinical Trial.
-
Central infusion of histamine reduces fat accumulation and upregulates UCP family in leptin-resistant obese mice.Diabetes. 2001 Feb;50(2):376-84. doi: 10.2337/diabetes.50.2.376. Diabetes. 2001. PMID: 11272150
-
Leptin signaling, adiposity, and energy balance.Ann N Y Acad Sci. 2002 Jun;967:379-88. doi: 10.1111/j.1749-6632.2002.tb04293.x. Ann N Y Acad Sci. 2002. PMID: 12079865 Review.
-
Clinical aspects of leptin.Vitam Horm. 1998;54:1-30. doi: 10.1016/s0083-6729(08)60919-x. Vitam Horm. 1998. PMID: 9529971 Review.
Cited by
-
Over-expression of leptin receptors in hypothalamic POMC neurons increases susceptibility to diet-induced obesity.PLoS One. 2012;7(1):e30485. doi: 10.1371/journal.pone.0030485. Epub 2012 Jan 20. PLoS One. 2012. PMID: 22276206 Free PMC article.
-
Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk.Cell Metab. 2016 May 10;23(5):770-84. doi: 10.1016/j.cmet.2016.04.011. Cell Metab. 2016. PMID: 27166942 Free PMC article. Review.
-
Intranasal treatment of central nervous system dysfunction in humans.Pharm Res. 2013 Oct;30(10):2475-84. doi: 10.1007/s11095-012-0915-1. Epub 2012 Nov 8. Pharm Res. 2013. PMID: 23135822 Free PMC article. Review.
-
Selective leptin resistance revisited.Am J Physiol Regul Integr Comp Physiol. 2013 Sep 15;305(6):R566-81. doi: 10.1152/ajpregu.00180.2013. Epub 2013 Jul 24. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23883674 Free PMC article. Review.
-
Optogenetic and Chemogenetic Approaches for Studying Astrocytes and Gliotransmitters.Exp Neurobiol. 2016 Oct;25(5):205-221. doi: 10.5607/en.2016.25.5.205. Epub 2016 Oct 20. Exp Neurobiol. 2016. PMID: 27790055 Free PMC article. Review.
References
-
- Adolph E F. Am J Physiol. 1947;151:110–125. - PubMed
-
- Hervey G R. Nature (London) 1969;222:629–631. - PubMed
-
- Leibel R L, Rosenbaum M, Hirsch J. N Engl J Med. 1995;332:621–628. - PubMed
-
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. - PubMed
-
- Tartaglia L A, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, Muir C, Sanker S, Moriarty A, Moore K, Smutko J S, Mays G G, Woolf E A, Monroe C A, Tepper R I. Cell. 1995;83:1263–1271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

