Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial, and postmitochondrial phases
- PMID: 9236228
- PMCID: PMC3913837
Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial, and postmitochondrial phases
Expression of concern in
-
Notice of concern: Ellerby et al., Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial, and postmitochondrial phases.J Neurosci. 2013 Oct 30;33(44):17549. doi: 10.1523/JNEUROSCI.4235-13.2013. J Neurosci. 2013. PMID: 24174687 Free PMC article. No abstract available.
Abstract
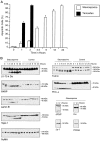
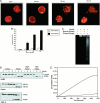
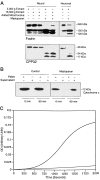
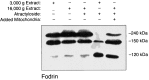
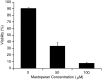
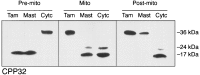
Apoptosis is a fundamental process required for normal development of the nervous system and is triggered during neurodegenerative disease. To dissect the molecular events leading to neuronal cell death, we have developed a cell-free model of neuronal apoptosis. The model faithfully reproduces key elements of apoptosis, including chromatin condensation, DNA fragmentation, caspase activation/processing, and selective substrate cleavage. We report that cell-free apoptosis is activated in premitochondrial, mitochondrial, and postmitochondrial phases by tamoxifen, mastoparan, and cytochrome c, respectively, allowing a functional ordering of these proapoptotic modulators. Furthermore, this is the first report of mitochondrial-mediated activation of cell-free apoptosis in a cell extract. Although Bcl-2 blocks activation at the premitochondrial and mitochondrial levels, it does not affect the postmitochondrial level. The cell-free system described here provides a valuable tool to elucidate the molecular events leading to neuronal cell death.
Figures


Similar articles
-
Glutamate-induced apoptosis in primary cortical neurons is inhibited by equine estrogens via down-regulation of caspase-3 and prevention of mitochondrial cytochrome c release.BMC Neurosci. 2005 Feb 24;6:13. doi: 10.1186/1471-2202-6-13. BMC Neurosci. 2005. PMID: 15730564 Free PMC article.
-
Cascade of caspase activation in potassium-deprived cerebellar granule neurons: targets for treatment with peptide and protein inhibitors of apoptosis.Mol Cell Neurosci. 2001 Apr;17(4):717-31. doi: 10.1006/mcne.2001.0962. Mol Cell Neurosci. 2001. PMID: 11312607
-
Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c.Cell. 1996 Jul 12;86(1):147-57. doi: 10.1016/s0092-8674(00)80085-9. Cell. 1996. PMID: 8689682
-
Apoptosis in neuronal cells: role of caspases.Neuroreport. 1998 Jul 13;9(10):R49-55. doi: 10.1097/00001756-199807130-00001. Neuroreport. 1998. PMID: 9694191 Review.
-
Sweet as honey, bitter as bile: Mitochondriotoxic peptides and other therapeutic proteins isolated from animal tissues, for dealing with mitochondrial apoptosis.Toxicology. 2021 Jan 15;447:152612. doi: 10.1016/j.tox.2020.152612. Epub 2020 Nov 7. Toxicology. 2021. PMID: 33171268 Review.
Cited by
-
Caspase inhibition protects nerve terminals from in vitro degradation.Neurochem Res. 2002 Jun;27(6):465-72. doi: 10.1023/a:1019840417796. Neurochem Res. 2002. PMID: 12199150
-
Dissection of the BCL-2 family signaling network with stabilized alpha-helices of BCL-2 domains.Methods Enzymol. 2008;446:387-408. doi: 10.1016/S0076-6879(08)01623-6. Methods Enzymol. 2008. PMID: 18603135 Free PMC article.
-
Skeletal muscle differentiation evokes endogenous XIAP to restrict the apoptotic pathway.PLoS One. 2009;4(3):e5097. doi: 10.1371/journal.pone.0005097. Epub 2009 Mar 31. PLoS One. 2009. PMID: 19333375 Free PMC article.
-
Death commitment point is advanced by axotomy in sympathetic neurons.J Cell Biol. 2000 Aug 21;150(4):741-54. doi: 10.1083/jcb.150.4.741. J Cell Biol. 2000. PMID: 10953000 Free PMC article.
-
Compartmental neurodegeneration and synaptic plasticity in the Wld(s) mutant mouse.J Physiol. 2001 Aug 1;534(Pt 3):627-39. doi: 10.1111/j.1469-7793.2001.00627.x. J Physiol. 2001. PMID: 11483696 Free PMC article. Review.
References
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
-
- Arnett TR, Lindsay R, Kilb JM, Moonga BS, Spowage M, Dempster DW. Selective toxic effects of tamoxifen on osteoclasts: comparison with the effects of estrogen. J Endocrinol. 1996;149:503–508. - PubMed
-
- Azi A, Montecucco C, Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals in biological membranes. Biochem Biophys Res Commun. 1975;65:597–603. - PubMed
-
- Bravo R, Frank R, Blundelli PA, McDonald-Bravo H. Cyclin-PCNA is the auxiliary protein of DNA polymerase delta. Nature. 1987;326:515–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
