Altered expression of the WT1 wilms tumor suppressor gene in human breast cancer
- PMID: 9223327
- PMCID: PMC21569
- DOI: 10.1073/pnas.94.15.8132
Altered expression of the WT1 wilms tumor suppressor gene in human breast cancer
Abstract
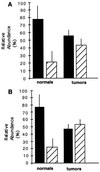
The product of the WT1 Wilms tumor suppressor gene controls the expression of genes encoding components of the insulin-like growth factor and transforming growth factor beta signaling systems. The role of these growth factors in breast tumor growth led us to investigate possible WT1 gene expression in normal and cancerous breast tissue. WT1 was detected by immunohistochemistry in the normal mammary duct and lobule, and the patterns of expression were consistent with developmental regulation. In a survey of 21 infiltrating tumors, 40% lacked immunodetectable WT1 altogether and an additional 28% were primarily WT1-negative. Cytoplasmic, but not nuclear, localization of WT1 was noted in some tumor cells and WT1 was detected, sometimes at high levels, in more-advanced estrogen-receptor-negative tumors. In this highly malignant subset, the tumor suppressor protein p53, which can physically interact with WT1, was also sometimes detected. WT1 mRNA was detected in normal and tumor tissue by reverse transcription-coupled PCR. Alternative splicing of the WT1 mRNA may regulate gene targeting of the WT1 protein through changes either in its regulatory or zinc-finger domains. The relative proportions of WT1 mRNA splice variants were altered in a random sample of breast tumors, providing evidence that different tumors may share a common WT1-related defect resulting in altered regulation of target genes.
Figures


Similar articles
-
High frequency of loss of allelic integrity at Wilms' tumor suppressor gene-1 locus in advanced breast tumors associated with aggressiveness of the tumor.Indian J Cancer. 2009 Oct-Dec;46(4):303-10. doi: 10.4103/0019-509X.55550. Indian J Cancer. 2009. PMID: 19749460
-
Regulated expression and potential roles of p53 and Wilms' tumor suppressor gene (WT1) during follicular development in the human ovary.J Clin Endocrinol Metab. 2000 Jan;85(1):449-59. doi: 10.1210/jcem.85.1.6246. J Clin Endocrinol Metab. 2000. PMID: 10634423
-
Functional properties of WT1.Med Pediatr Oncol. 1996 Nov;27(5):453-5. doi: 10.1002/(SICI)1096-911X(199611)27:5<453::AID-MPO11>3.0.CO;2-B. Med Pediatr Oncol. 1996. PMID: 8827073
-
Wilms tumor and the WT1 gene.Exp Cell Res. 2001 Mar 10;264(1):74-99. doi: 10.1006/excr.2000.5131. Exp Cell Res. 2001. PMID: 11237525 Review.
-
Multiple roles for the Wilms' tumor suppressor, WT1.Cancer Res. 1999 Apr 1;59(7 Suppl):1747s-1750s; discussion 1751s. Cancer Res. 1999. PMID: 10197591 Review.
Cited by
-
Opposite regulation of estrogen receptor-α and its variant ER-α36 by the Wilms' tumor suppressor WT1.Oncol Lett. 2011;2(2):337-341. doi: 10.3892/ol.2011.250. Epub 2011 Jan 21. Oncol Lett. 2011. PMID: 22737185 Free PMC article.
-
Expression of Wilms' tumor gene (WT1) is associated with survival in malignant pleural mesothelioma.Clin Transl Oncol. 2014 Sep;16(9):776-82. doi: 10.1007/s12094-013-1146-6. Epub 2013 Dec 10. Clin Transl Oncol. 2014. PMID: 24323466
-
Wilms' tumor 1 (WT1) as a prognosis factor in gynecological cancers: A meta-analysis.Medicine (Baltimore). 2018 Jul;97(28):e11485. doi: 10.1097/MD.0000000000011485. Medicine (Baltimore). 2018. PMID: 29995811 Free PMC article.
-
Prognostic significance of WT1 expression in soft tissue sarcoma.World J Surg Oncol. 2014 Jul 16;12:214. doi: 10.1186/1477-7819-12-214. World J Surg Oncol. 2014. PMID: 25026998 Free PMC article.
-
Methylation of WT1, CA10 in peripheral blood leukocyte is associated with breast cancer risk: a case-control study.BMC Cancer. 2020 Jul 31;20(1):713. doi: 10.1186/s12885-020-07183-8. BMC Cancer. 2020. PMID: 32736539 Free PMC article.
References
-
- Ruan W, Catanese V, Wieczorek R, Feldman M, Kleinberg D. Endocrinology. 1995;136:1296–1302. - PubMed
-
- Yee D. Breast Cancer Res Treat. 1994;32:85–95. - PubMed
-
- Lee A, Yee D. Biomed Pharmacother. 1995;49:415–421. - PubMed
-
- Peyrat J, Bonneterre J. Breast Cancer Res Treat. 1992;22:59–67. - PubMed
-
- Brunner N, Yee D, Kern F, Spang-Thomsen M, Lippman M, Cullen K. Eur J Cancer. 1993;29A:562–569. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

