A highly temperature-sensitive proton current in mouse bone marrow-derived mast cells
- PMID: 9222899
- PMCID: PMC2217037
- DOI: 10.1085/jgp.109.6.731
A highly temperature-sensitive proton current in mouse bone marrow-derived mast cells
Abstract
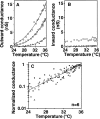
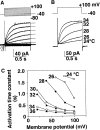
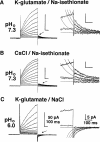
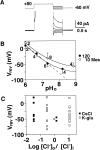
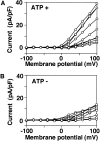
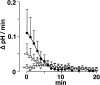
Proton (H+) conductive pathways are suggested to play roles in the regulation of intracellular pH. We characterized temperature-sensitive whole cell currents in mouse bone marrow-derived mast cells (BMMC), immature proliferating mast cells generated by in vitro culture. Heating from 24 to 36 degrees C reversibly and repeatedly activated a voltage-dependent outward conductance with Q10 of 9.9 +/- 3.1 (mean +/- SD) (n = 6). Either a decrease in intracellular pH or an increase in extracellular pH enhanced the amplitude and shifted the activation voltage to more negative potentials. With acidic intracellular solutions (pH 5.5), the outward current was detected in some cells at 24 degrees C and Q10 was 6.0 +/- 2.6 (n = 9). The reversal potential was unaffected by changes in concentrations of major ionic constituents (K+, Cl-, and Na+), but depended on the pH gradient, suggesting that H+ (equivalents) is a major ion species carrying the current. The H+ current was featured by slow activation kinetics upon membrane depolarization, and the activation time course was accelerated by increases in depolarization, elevating temperature and extracellular alkalization. The current was recorded even when ATP was removed from the intracellular solution, but the mean amplitude was smaller than that in the presence of ATP. The H+ current was reversibly inhibited by Zn2+ but not by bafilomycin A1, an inhibitor for a vacuolar type H(+)-ATPase. Macroscopic measurements of pH using a fluorescent dye (BCECF) revealed that a rapid recovery of intracellular pH from acid-load was attenuated by lowering temperature, addition of Zn2+, and depletion of extracellular K+, but not by bafilomycin A1. These results suggest that the H+ conductive pathway contributes to intracellular pH homeostasis of BMMC and that the high activation energy may be involved in enhancement of the H+ conductance.
Figures

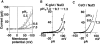
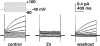

Similar articles
-
pH dependence and inhibition by extracellular calcium of proton currents via plasmalemmal vacuolar-type H+-ATPase in murine osteoclasts.J Physiol. 2006 Oct 15;576(Pt 2):417-25. doi: 10.1113/jphysiol.2006.117176. Epub 2006 Aug 10. J Physiol. 2006. PMID: 16901940 Free PMC article.
-
A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages.J Gen Physiol. 1993 Oct;102(4):729-60. doi: 10.1085/jgp.102.4.729. J Gen Physiol. 1993. PMID: 8270911 Free PMC article.
-
A heterogeneous electrophysiological profile of bone marrow-derived mast cells.J Membr Biol. 1995 Jan;143(2):115-22. doi: 10.1007/BF00234657. J Membr Biol. 1995. PMID: 7731031
-
Potential contribution of a voltage-activated proton conductance to acid extrusion from rat hippocampal neurons.Neuroscience. 2008 Feb 19;151(4):1084-98. doi: 10.1016/j.neuroscience.2007.12.007. Epub 2007 Dec 8. Neuroscience. 2008. PMID: 18201832
-
Regulation of cytoplasmic pH in osteoclasts. Contribution of proton pumps and a proton-selective conductance.J Biol Chem. 1995 Feb 3;270(5):2203-12. doi: 10.1074/jbc.270.5.2203. J Biol Chem. 1995. PMID: 7836451
Cited by
-
Depolarization of isolated horizontal cells of fish acidifies their immediate surrounding by activating V-ATPase.J Physiol. 2007 Dec 1;585(Pt 2):401-12. doi: 10.1113/jphysiol.2007.142646. Epub 2007 Oct 11. J Physiol. 2007. PMID: 17932147 Free PMC article.
-
NOX family NADPH oxidases: do they have built-in proton channels?J Gen Physiol. 2002 Dec;120(6):781-6. doi: 10.1085/jgp.20028713. J Gen Physiol. 2002. PMID: 12451048 Free PMC article. Review. No abstract available.
-
Voltage-gated proton channels: what's next?J Physiol. 2008 Nov 15;586(22):5305-24. doi: 10.1113/jphysiol.2008.161703. Epub 2008 Sep 18. J Physiol. 2008. PMID: 18801839 Free PMC article. Review.
-
Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement.J Gen Physiol. 2003 Jun;121(6):615-28. doi: 10.1085/jgp.200308813. J Gen Physiol. 2003. PMID: 12771195 Free PMC article.
-
Early and late activation of the voltage-gated proton channel during lactic acidosis through pH-dependent and -independent mechanisms.Pflugers Arch. 2008 Feb;455(5):829-38. doi: 10.1007/s00424-007-0339-7. Epub 2007 Sep 18. Pflugers Arch. 2008. PMID: 17876602
References
-
- Alfonso A, Botana MA, Vieytes MR, Louzao MC, Botana LM. Effect of signal transduction pathways on the action of thapsigargin on rat mast cells: crosstalks between cellular signaling and cytosolic pH. Biochem Pharmacol. 1994;47:1813–1820. - PubMed

