Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation
- PMID: 9204920
- PMCID: PMC6793827
- DOI: 10.1523/JNEUROSCI.17-14-05357.1997
Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation
Abstract
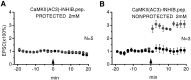
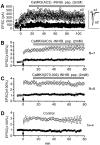
The role of postsynaptic kinases in the induction and maintenance of long-term potentiation (LTP) was studied in the CA1 region of the rat hippocampal slice. A peptide inhibitor for the catalytic domain of calcium/calmodulin-dependent protein kinase type II (CaM-kinase) was applied through a perfused patch pipette. The inhibitor completely blocked both the short-term potentiation and LTP induced by a pairing protocol. This indicates that the kinase or kinases affected by the peptide are downstream from depolarization in the LTP cascade. The ability to block LTP required that measures be taken to interfere with degradation of the peptide kinase inhibitor by endogenous proteases; either addition of protease inhibitors or modifications of the peptide itself greatly enhanced the effectiveness of the peptide. Protease inhibitors by themselves or control peptide did not block LTP induction. To study the effect of kinase inhibitor on LTP maintenance, we induced LTP in one pathway. Subsequent introduction of the kinase inhibitor blocked the induction of LTP in a second pathway, but it did not affect maintenance of LTP in the first. The implications for the role of kinases in LTP maintenance are discussed.
Figures

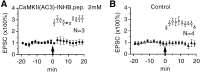



Similar articles
-
Attenuation of paired-pulse facilitation associated with synaptic potentiation mediated by postsynaptic mechanisms.J Neurophysiol. 1997 Nov;78(5):2707-16. doi: 10.1152/jn.1997.78.5.2707. J Neurophysiol. 1997. PMID: 9356420
-
Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor.J Neurosci. 2007 May 9;27(19):5190-9. doi: 10.1523/JNEUROSCI.5049-06.2007. J Neurosci. 2007. PMID: 17494705 Free PMC article.
-
Ca2+/calmodulin-dependent protein kinase II-dependent long-term potentiation in the rat suprachiasmatic nucleus and its inhibition by melatonin.J Neurosci Res. 2002 Dec 15;70(6):799-807. doi: 10.1002/jnr.10400. J Neurosci Res. 2002. PMID: 12444602
-
CaM kinase II in long-term potentiation.Neurochem Int. 1996 Apr;28(4):343-58. doi: 10.1016/0197-0186(95)00097-6. Neurochem Int. 1996. PMID: 8740440 Review.
-
A role of Ca2+/calmodulin-dependent protein kinase II in the induction of long-term potentiation in hippocampal CA1 area.Neurosci Res. 1996 Jan;24(2):117-22. doi: 10.1016/0168-0102(95)00991-4. Neurosci Res. 1996. PMID: 8929917 Review.
Cited by
-
KIBRA anchoring the action of PKMζ maintains the persistence of memory.Sci Adv. 2024 Jun 28;10(26):eadl0030. doi: 10.1126/sciadv.adl0030. Epub 2024 Jun 26. Sci Adv. 2024. PMID: 38924398 Free PMC article.
-
The high variance of AMPA receptor- and NMDA receptor-mediated responses at single hippocampal synapses: evidence for multiquantal release.Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4885-90. doi: 10.1073/pnas.0630290100. Epub 2003 Apr 7. Proc Natl Acad Sci U S A. 2003. PMID: 12682300 Free PMC article.
-
Inhibition of the cAMP pathway decreases early long-term potentiation at CA1 hippocampal synapses.J Neurosci. 2000 Jun 15;20(12):4446-51. doi: 10.1523/JNEUROSCI.20-12-04446.2000. J Neurosci. 2000. PMID: 10844013 Free PMC article.
-
Tag-trigger-consolidation: a model of early and late long-term-potentiation and depression.PLoS Comput Biol. 2008 Dec;4(12):e1000248. doi: 10.1371/journal.pcbi.1000248. Epub 2008 Dec 26. PLoS Comput Biol. 2008. PMID: 19112486 Free PMC article.
-
Novel Selective Calpain 1 Inhibitors as Potential Therapeutics in Alzheimer's Disease.J Alzheimers Dis. 2016;49(3):707-21. doi: 10.3233/JAD-150618. J Alzheimers Dis. 2016. PMID: 26484927 Free PMC article.
References
-
- Barria A, Muller D, Griffith LC, Soderling TR (1997) Phosphorylation of AMPA-type glutamate receptors by Ca2+/calmodulin-dependent protein kinase II during long-term potentiation. Science, in press. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. - PubMed
-
- Bond JS, Butler PE. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
