Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles
- PMID: 9199166
- PMCID: PMC2137815
- DOI: 10.1083/jcb.137.7.1495
Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles
Abstract
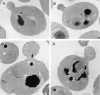
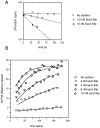
The small GTPase Sec4p is required for vesicular transport at the post-Golgi stage of yeast secretion. Here we present evidence that mutations in SEC2, itself an essential gene that acts at the same stage of the secretory pathway, cause Sec4p to mislocalize as a result of a random rather than a polarized accumulation of vesicles. Sec2p and Sec4p interact directly, with the nucleotide-free conformation of Sec4p being the preferred state for interaction with Sec2p. Sec2p functions as an exchange protein, catalyzing the dissociation of GDP from Sec4 and promoting the binding of GTP. We propose that Sec2p functions to couple the activation of Sec4p to the polarized delivery of vesicles to the site of exocytosis.
Figures




Similar articles
-
Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast.J Cell Biol. 2002 Jun 10;157(6):1005-15. doi: 10.1083/jcb.200201003. Epub 2002 Jun 3. J Cell Biol. 2002. PMID: 12045183 Free PMC article.
-
The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles.J Cell Biol. 2000 Apr 3;149(1):95-110. doi: 10.1083/jcb.149.1.95. J Cell Biol. 2000. PMID: 10747090 Free PMC article.
-
Interactions of nucleotide release factor Dss4p with Sec4p in the post-Golgi secretory pathway of yeast.J Biol Chem. 1997 Jul 18;272(29):18281-9. doi: 10.1074/jbc.272.29.18281. J Biol Chem. 1997. PMID: 9218467
-
The cycle of SEC4 function in vesicular transport.Ciba Found Symp. 1993;176:218-28; discussion 229-32. Ciba Found Symp. 1993. PMID: 8299422 Review.
-
Small GTP-binding proteins and their role in transport.Curr Opin Cell Biol. 1991 Aug;3(4):626-33. doi: 10.1016/0955-0674(91)90033-u. Curr Opin Cell Biol. 1991. PMID: 1663370 Review. No abstract available.
Cited by
-
Structural basis for recognition of the Sec4 Rab GTPase by its effector, the Lgl/tomosyn homologue, Sro7.Mol Biol Cell. 2015 Sep 15;26(18):3289-300. doi: 10.1091/mbc.E15-04-0228. Epub 2015 Jul 22. Mol Biol Cell. 2015. PMID: 26202462 Free PMC article.
-
Exocyst sec5 regulates exocytosis of newcomer insulin granules underlying biphasic insulin secretion.PLoS One. 2013 Jul 2;8(7):e67561. doi: 10.1371/journal.pone.0067561. Print 2013. PLoS One. 2013. PMID: 23844030 Free PMC article.
-
Two distinct regions in a yeast myosin-V tail domain are required for the movement of different cargoes.J Cell Biol. 2000 Aug 7;150(3):513-26. doi: 10.1083/jcb.150.3.513. J Cell Biol. 2000. PMID: 10931864 Free PMC article.
-
Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors.J Cell Biol. 2010 Oct 18;191(2):367-81. doi: 10.1083/jcb.201008051. Epub 2010 Oct 11. J Cell Biol. 2010. PMID: 20937701 Free PMC article.
-
The exocyst complex in exocytosis and cell migration.Protoplasma. 2012 Jul;249(3):587-97. doi: 10.1007/s00709-011-0330-1. Epub 2011 Oct 14. Protoplasma. 2012. PMID: 21997494 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

