A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers
- PMID: 9192659
- PMCID: PMC21252
- DOI: 10.1073/pnas.94.13.6874
A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers
Abstract
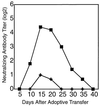
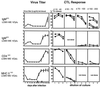
This study demonstrates that neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons (IFNs) are of key importance in virus control both in adoptive immunotherapy of persistent infection and in the late phase of acute infection with the WE strain of lymphocytic choriomeningitis virus (LCMV). We report the following results. (i) Clearance of LCMV-WE from C57BL/6 carrier mice by adoptive transfer of memory spleen cells requires B cells and CD4(+) T cells but not necessarily CD8(+) T cells. (ii) At the doses examined, CD8(+) T cells contribute to the initial reduction of viral titers but are alone not sufficient to clear the virus because they are exhausted. (iii) In the presence of functional IFN-gamma, virus clearance correlates well with the generation of neutralizing antibodies in the treated carrier mice. (iv) In the absence of receptors for IFN-gamma, virus clearance is not achieved. (v) Adoptive immunotherapy of mice persistently infected with a distinct virus isolate, LCMV-Armstrong, revealed only low levels of neutralizing antibodies; in this case, CD8(+) T cells were needed for virus clearance in addition to B and CD4(+) T cells. (vi) After low dose infection of C57BL/6 mice with LCMV-WE, virus is eliminated below detectable levels by CD8(+) T cells, but long-term (>2 months) virus control is usually not achieved in the absence of B cells or CD4(+) T cells; reappearance of the virus is paralleled either by exhaustion of virus-specific cytotoxic T lymphocytes or lethal immunopathology. These findings are of importance for adoptive immunotherapy strategies against persistent virus infections in humans.
Figures

Similar articles
-
Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells.J Virol. 2001 Sep;75(18):8407-23. doi: 10.1128/jvi.75.18.8407-8423.2001. J Virol. 2001. PMID: 11507186 Free PMC article.
-
Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice.J Virol. 1998 Nov;72(11):9208-16. doi: 10.1128/JVI.72.11.9208-9216.1998. J Virol. 1998. PMID: 9765468 Free PMC article.
-
Exhaustion of cytotoxic T cells during adoptive immunotherapy of virus carrier mice can be prevented by B cells or CD4+ T cells.Eur J Immunol. 2002 Feb;32(2):374-82. doi: 10.1002/1521-4141(200202)32:2<374::AID-IMMU374>3.0.CO;2-9. Eur J Immunol. 2002. PMID: 11813156
-
Complexities of Type I Interferon Biology: Lessons from LCMV.Viruses. 2019 Feb 20;11(2):172. doi: 10.3390/v11020172. Viruses. 2019. PMID: 30791575 Free PMC article. Review.
-
Viral escape from the neutralizing antibody response: the lymphocytic choriomeningitis virus model.Immunogenetics. 2001 Apr;53(3):185-9. doi: 10.1007/s002510100314. Immunogenetics. 2001. PMID: 11398962 Review.
Cited by
-
IgM Antibody Repertoire Fingerprints in Mice Are Personalized but Robust to Viral Infection Status.Front Cell Infect Microbiol. 2020 May 28;10:254. doi: 10.3389/fcimb.2020.00254. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32547966 Free PMC article.
-
Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells.J Virol. 2001 Sep;75(18):8407-23. doi: 10.1128/jvi.75.18.8407-8423.2001. J Virol. 2001. PMID: 11507186 Free PMC article.
-
Transient antiretroviral treatment during acute simian immunodeficiency virus infection facilitates long-term control of the virus.Philos Trans R Soc Lond B Biol Sci. 2000 Aug 29;355(1400):1021-9. doi: 10.1098/rstb.2000.0639. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11186303 Free PMC article. Review.
-
Progress in defining CD4 helper cell responses in chronic viral infections.J Exp Med. 2003 Dec 15;198(12):1773-7. doi: 10.1084/jem.20031947. J Exp Med. 2003. PMID: 14676292 Free PMC article. No abstract available.
-
Lymphocytic choriomeningitis infection of the central nervous system.Front Biosci. 2008 May 1;13:4529-43. doi: 10.2741/3021. Front Biosci. 2008. PMID: 18508527 Free PMC article. Review.
References
-
- Cole G A, Nathanson N, Prendergast R A. Nature (London) 1972;238:335–337. - PubMed
-
- Doherty P C, Zinkernagel R M. Transplant Rev. 1974;19:89–120. - PubMed
-
- Zinkernagel R M, Welsh R M. J Immunol. 1976;117:1495–1502. - PubMed
-
- Hotchin J. Cold Spring Harbor Symp Quant Biol. 1962;27:479–501. - PubMed
-
- Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. Adv Immunol. 1980;30:275–331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

