Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain
- PMID: 9185542
- PMCID: PMC6573289
- DOI: 10.1523/JNEUROSCI.17-13-05046.1997
Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain
Abstract
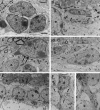
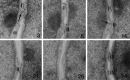
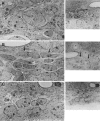
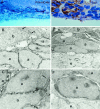
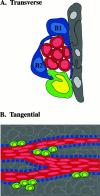
The adult mammalian subventricular zone (SVZ) contains stem cells that give rise to neurons and glia. In vivo, SVZ progeny migrate 3-8 mm to the olfactory bulb, where they form neurons. We show here that the SVZ of the lateral wall of the lateral ventricles in adult mice is composed of neuroblasts, glial cells, and a novel putative precursor cell. The topographical organization of these cells suggests how neurogenesis and migration are integrated in this region. Type A cells had the ultrastructure of migrating neuronal precursors. These cells were arranged as chains parallel to the walls of the ventricle and were polysialylated neural adhesion cell molecule- (PSA-NCAM), TuJ1- (beta-tubulin), and nestin-positive but GFAP- and vimentin-negative. Chains of Type A cells were ensheathed by two ultrastructurally distinct astrocytes (Type B1 and B2) that were GFAP-, vimentin-, and nestin-positive but PSA-NCAM- and TuJ1-negative. Type A and B2 (but not B1) cells incorporated [3H]thymidine. The most actively dividing cell in the SVZ corresponded to Type C cells, which had immature ultrastructural characteristics and were nestin-positive but negative to the other markers. Type C cells formed focal clusters closely associated with chains of Type A cells. Whereas Type C cells were present throughout the SVZ, they were not found in the rostral migratory stream that links the SVZ with the olfactory bulb. These results suggest that chains of migrating neuroblasts in the SVZ may be derived from Type C cells. Our results provide a topographical model for the adult SVZ and should serve as a basis for the in vivo identification of stem cells in the adult mammalian brain.
Figures




Similar articles
-
Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice.J Comp Neurol. 1995 Jan 2;351(1):51-61. doi: 10.1002/cne.903510106. J Comp Neurol. 1995. PMID: 7896939
-
Expression of trophinin and bystin identifies distinct cell types in the germinal zones of adult rat brain.Eur J Neurosci. 2006 May;23(9):2265-76. doi: 10.1111/j.1460-9568.2006.04782.x. Eur J Neurosci. 2006. PMID: 16706835
-
Characterization of the subventricular zone of the adult human brain: evidence for the involvement of Bcl-2.Neurosci Res. 2000 May;37(1):67-78. doi: 10.1016/s0168-0102(00)00102-4. Neurosci Res. 2000. PMID: 10802345
-
Architecture and cell types of the adult subventricular zone: in search of the stem cells.J Neurobiol. 1998 Aug;36(2):234-48. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. J Neurobiol. 1998. PMID: 9712307 Review.
-
The human brain subventricular zone: stem cells in this niche and its organization.Neurosurg Clin N Am. 2007 Jan;18(1):15-20, vii. doi: 10.1016/j.nec.2006.10.013. Neurosurg Clin N Am. 2007. PMID: 17244550 Review.
Cited by
-
Roles of reactive oxygen species in the fate of stem cells.Antioxid Redox Signal. 2014 Apr 20;20(12):1881-90. doi: 10.1089/ars.2012.4963. Epub 2012 Nov 19. Antioxid Redox Signal. 2014. PMID: 23066813 Free PMC article. Review.
-
Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone.Cell Prolif. 2013 Apr;46(2):137-45. doi: 10.1111/cpr.12023. Cell Prolif. 2013. PMID: 23510468 Free PMC article.
-
Maturation of postnatally generated olfactory bulb granule cells depends on functional γ-protocadherin expression.Sci Rep. 2013;3:1514. doi: 10.1038/srep01514. Sci Rep. 2013. PMID: 23515096 Free PMC article.
-
Enhanced lithium-induced brain recovery following cranial irradiation is not impeded by inflammation.Stem Cells Transl Med. 2012 Jun;1(6):469-79. doi: 10.5966/sctm.2011-0046. Epub 2012 May 30. Stem Cells Transl Med. 2012. PMID: 23197851 Free PMC article.
-
Experimental Advances Towards Neural Regeneration from Induced Stem Cells to Direct In Vivo Reprogramming.Mol Neurobiol. 2016 May;53(4):2124-31. doi: 10.1007/s12035-015-9181-7. Epub 2015 May 2. Mol Neurobiol. 2016. PMID: 25934102 Review.
References
-
- Allen E. The cessation of mitosis in the central nervous system of the albino rat. J Comp Neurol. 1912;22:547–568.
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–458. - PubMed
-
- Alvarez-Buylla A, Lois C. Neuronal stem cells in the brain of adult vertebrates. Stem Cells. 1995;13:263–272. - PubMed
-
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264:159–170. - PubMed
-
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
