Gsalpha contains an unidentified covalent modification that increases its affinity for adenylyl cyclase
- PMID: 9177179
- PMCID: PMC21011
- DOI: 10.1073/pnas.94.12.6116
Gsalpha contains an unidentified covalent modification that increases its affinity for adenylyl cyclase
Abstract
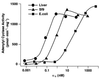
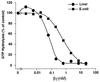
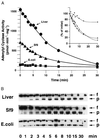
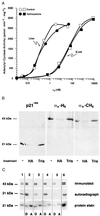
Many G protein alpha subunits are dually acylated with myristate and palmitate or are palmitoylated on more than one cysteine residue near their N termini. The Galpha protein that activates adenylyl cyclase, alphas, is not myristoylated but can be reversibly palmitoylated. It appears that alphas contains another, as-yet-unidentified covalent modification that decreases its apparent dissociation constant for adenylyl cyclase from 50 nM to <0. 5 nM. This modification is at or near the N terminus of the protein and is hydrophobic. Palmitoylation of native alphas does not account for its high affinity for adenylyl cyclase.
Figures

Similar articles
-
Conditional activation defect of a human Gsalpha mutant.Proc Natl Acad Sci U S A. 1997 May 27;94(11):5656-61. doi: 10.1073/pnas.94.11.5656. Proc Natl Acad Sci U S A. 1997. PMID: 9159128 Free PMC article.
-
Purification of recombinant G proteins from Sf9 cells by hexahistidine tagging of associated subunits. Characterization of alpha 12 and inhibition of adenylyl cyclase by alpha z.J Biol Chem. 1995 Jan 27;270(4):1734-41. doi: 10.1074/jbc.270.4.1734. J Biol Chem. 1995. PMID: 7829508
-
Phorbol ester-induced stimulation and phosphorylation of adenylyl cyclase 2.Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10630-4. doi: 10.1073/pnas.91.22.10630. Proc Natl Acad Sci U S A. 1994. PMID: 7938004 Free PMC article.
-
[Receptor-adenylate cyclase coupling--receptor-GTP binding protein(N) coupling and ADP-ribosylation of N by bacterial toxins].Tanpakushitsu Kakusan Koso. 1985 Apr;30(4):303-13. Tanpakushitsu Kakusan Koso. 1985. PMID: 2861624 Review. Japanese. No abstract available.
-
Regulation of mammalian adenylyl cyclases by G-protein alpha and beta gamma subunits.Cold Spring Harb Symp Quant Biol. 1992;57:135-44. doi: 10.1101/sqb.1992.057.01.017. Cold Spring Harb Symp Quant Biol. 1992. PMID: 1339652 Review. No abstract available.
Cited by
-
Plasma membrane localization of G alpha z requires two signals.Mol Biol Cell. 1998 Jan;9(1):1-14. doi: 10.1091/mbc.9.1.1. Mol Biol Cell. 1998. PMID: 9436987 Free PMC article.
-
Galpha(s) is palmitoylated at the N-terminal glycine.EMBO J. 2003 Feb 17;22(4):826-32. doi: 10.1093/emboj/cdg095. EMBO J. 2003. PMID: 12574119 Free PMC article.
References
-
- Casey P J. Science. 1995;268:221–225. - PubMed
-
- Wedegaertner P B, Wilson P T, Bourne H R. J Biol Chem. 1995;270:503–506. - PubMed
-
- Mumby S M. Curr Opin Cell Biol. 1997;9:148–154. - PubMed
-
- Wall M A, Coleman D E, Lee E, Iñiguez-Lluhi J A, Posner B A, Gilman A G, Sprang S R. Cell. 1995;83:1047–1058. - PubMed
-
- Lambright D G, Sondek J, Bohm A, Skiba N P, Hamm H E, Sigler P B. Nature (London) 1996;379:311–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

