Regulation of interleukin-12 by complement receptor 3 signaling
- PMID: 9166428
- PMCID: PMC2196332
- DOI: 10.1084/jem.185.11.1987
Regulation of interleukin-12 by complement receptor 3 signaling
Abstract
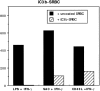
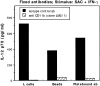
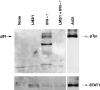
Complement receptor type 3 (CR3, CD11b/CD18) serves as a receptor for a number of endogenous ligands and infectious organisms, and is involved in adhesion and host defense functions. Here, we report that signaling via CR3 plays an important role in regulating production of interleukin-12 (IL-12), a key mediator of cell-mediated immunity (CMI). We demonstrate with a variety of stimuli a dose-dependent, specific downregulation of IL-12 secretion by human monocytes in vitro after exposure to antibodies to CR3 (anti-CD11b and anti-CD18), as well as to the natural CR3 ligands, iC3b, and Histoplasma capsulatum. CR3 antibodies also suppressed interferon-gamma (IFN-gamma) production in cultures of human peripheral blood mononuclear cells (PBMC). We determined that one mechanism by which CR3 antibodies may suppress IL-12 production is by the inhibition of IFN-gamma-induced tyrosine phosphorylation. Finally, in a murine model of IL-12-dependent septic shock, we provide evidence that administration of CR3 antibodies leads to suppression of IL-12 and IFN-gamma in vivo. Our studies thus define a novel role for CR3 in regulating CMI functions via IL-12.
Figures

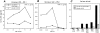

Similar articles
-
Leishmania major inhibits IL-12 in macrophages by signalling through CR3 (CD11b/CD18) and down-regulation of ETS-mediated transcription.Parasite Immunol. 2013 Dec;35(12):409-20. doi: 10.1111/pim.12049. Parasite Immunol. 2013. PMID: 23834512 Free PMC article.
-
The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes.Immunol Lett. 2017 Sep;189:64-72. doi: 10.1016/j.imlet.2017.05.014. Epub 2017 May 26. Immunol Lett. 2017. PMID: 28554712
-
Beta-glucan, a "specific" biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18).J Immunol. 1999 Sep 15;163(6):3045-52. J Immunol. 1999. PMID: 10477568
-
Contribution of CR3, CD11b/CD18 to cytolysis by human NK cells.Mol Immunol. 1990 Dec;27(12):1343-7. doi: 10.1016/0161-5890(90)90041-w. Mol Immunol. 1990. PMID: 1980339 Review.
-
CR3: a general purpose adhesion-recognition receptor essential for innate immunity.Microbes Infect. 2000 Mar;2(3):289-94. doi: 10.1016/s1286-4579(00)00299-9. Microbes Infect. 2000. PMID: 10758405 Review.
Cited by
-
Antigen-presenting dendritic cells rescue CD4-depleted CCR2-/- mice from lethal Histoplasma capsulatum infection.Infect Immun. 2010 May;78(5):2125-37. doi: 10.1128/IAI.00065-10. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194586 Free PMC article.
-
Resistance of Mycoplasma pulmonis to complement lysis is dependent on the number of Vsa tandem repeats: shield hypothesis.Infect Immun. 2004 Dec;72(12):6846-51. doi: 10.1128/IAI.72.12.6846-6851.2004. Infect Immun. 2004. PMID: 15557605 Free PMC article.
-
Conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages.Infect Immun. 2010 Sep;78(9):3871-82. doi: 10.1128/IAI.00204-10. Epub 2010 Jul 6. Infect Immun. 2010. PMID: 20605974 Free PMC article.
-
Flying under the radar: Histoplasma capsulatum avoidance of innate immune recognition.Semin Cell Dev Biol. 2019 May;89:91-98. doi: 10.1016/j.semcdb.2018.03.009. Epub 2018 Mar 21. Semin Cell Dev Biol. 2019. PMID: 29551572 Free PMC article. Review.
-
Graft rejection - endogenous or allogeneic?Immunology. 2012 Jun;136(2):123-32. doi: 10.1111/j.1365-2567.2012.03560.x. Immunology. 2012. PMID: 22260525 Free PMC article. Review.
References
-
- Springer TA. Adhesion receptors of the immune system. Nature (Lond) 1990;346:425–434. - PubMed
-
- Patarroyo M. Leukocyte adhesion to cells. Molecular basis, physiological relevance, and abnormalities. Scand J Immunol. 1989;30:129–164. - PubMed
-
- Cooper NR. Complement evasion strategies of microorganisms. Immunol Today. 1991;12:327–331. - PubMed
-
- Kishimoto TK, Larson RS, Corbi AL, Dustin ML, Staunton DE, Springer TA. The leukocyte integrins. Adv Immunol. 1989;46:149–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

