Conditional activation defect of a human Gsalpha mutant
- PMID: 9159128
- PMCID: PMC20834
- DOI: 10.1073/pnas.94.11.5656
Conditional activation defect of a human Gsalpha mutant
Abstract
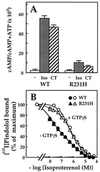
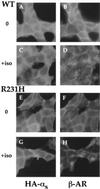
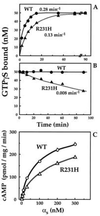
Hormonal signals activate trimeric G proteins by promoting exchange of GTP for GDP bound to the G protein's alpha subunit (Galpha). Here we describe a point mutation that impairs this activation mechanism in the alpha subunit of Gs, producing an inherited disorder of hormone responsiveness. Biochemical analysis reveals an activation defect that is paradoxically intensified by hormonal and other stimuli. By substituting histidine for a conserved arginine residue, the mutation removes an internal salt bridge (to a conserved glutamate) that normally acts as an intramolecular hasp to maintain tight binding of the gamma-phosphate of GTP. In its basal, unperturbed state, the mutant alphas binds guanosine 5'-[gamma-thio]triphosphate (GTP[gammaS]), a GTP analog, slightly less tightly than does normal alphas, but (in the GTP[gammaS]-bound form) can stimulate adenylyl cyclase. The activation defect becomes prominent only under conditions that destabilize binding of guanine nucleotide (receptor stimulation) or impair the ability of alphas to bind the gamma-phosphate of GTP (cholera toxin, AlF4- ion). Although GDP release is usually the rate-limiting step in nucleotide exchange, the biochemical phenotype of this mutant alphas indicates that efficient G protein activation by receptors and other stimuli depends on the ability of Galpha to clasp tightly the GTP molecule that enters the binding site.
Figures

Similar articles
-
Reconstitution of beta2-adrenoceptor-GTP-binding-protein interaction in Sf9 cells--high coupling efficiency in a beta2-adrenoceptor-G(s alpha) fusion protein.Eur J Biochem. 1998 Jul 15;255(2):369-82. doi: 10.1046/j.1432-1327.1998.2550369.x. Eur J Biochem. 1998. PMID: 9716378
-
A mutation in the heterotrimeric stimulatory guanine nucleotide binding protein alpha-subunit with impaired receptor-mediated activation because of elevated GTPase activity.Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4268-72. doi: 10.1073/pnas.96.8.4268. Proc Natl Acad Sci U S A. 1999. PMID: 10200251 Free PMC article.
-
The olfactory G protein G(alphaolf) possesses a lower GDP-affinity and deactivates more rapidly than G(salphashort): consequences for receptor-coupling and adenylyl cyclase activation.J Neurochem. 2001 Jul;78(2):325-38. doi: 10.1046/j.1471-4159.2001.00422.x. J Neurochem. 2001. PMID: 11461968
-
The complex regulation of receptor-coupled G-proteins.Adv Enzyme Regul. 1997;37:427-35. doi: 10.1016/s0065-2571(96)00020-9. Adv Enzyme Regul. 1997. PMID: 9381985 Review.
-
Cholera toxin.Biosci Rep. 1982 Mar;2(3):135-46. doi: 10.1007/BF01116376. Biosci Rep. 1982. PMID: 6121589 Review. No abstract available.
Cited by
-
Regulation of RhoA signaling by the cAMP-dependent phosphorylation of RhoGDIα.J Biol Chem. 2012 Nov 9;287(46):38705-15. doi: 10.1074/jbc.M112.401547. Epub 2012 Sep 25. J Biol Chem. 2012. PMID: 23012358 Free PMC article.
-
Molecular annotation of G protein variants in a neurological disorder.Cell Rep. 2023 Dec 26;42(12):113462. doi: 10.1016/j.celrep.2023.113462. Epub 2023 Nov 18. Cell Rep. 2023. PMID: 37980565 Free PMC article.
-
Analysis of the V2 Vasopressin Receptor (V2R) Mutations Causing Partial Nephrogenic Diabetes Insipidus Highlights a Sustainable Signaling by a Non-peptide V2R Agonist.J Biol Chem. 2016 Oct 21;291(43):22460-22471. doi: 10.1074/jbc.M116.733220. Epub 2016 Sep 6. J Biol Chem. 2016. PMID: 27601473 Free PMC article.
-
G protein β4 as a structural determinant of enhanced nucleotide exchange in the A2AAR-Gs complex.Res Sq [Preprint]. 2024 Jan 23:rs.3.rs-3814988. doi: 10.21203/rs.3.rs-3814988/v1. Res Sq. 2024. PMID: 38343806 Free PMC article. Preprint.
-
V2 vasopressin receptor (V2R) mutations in partial nephrogenic diabetes insipidus highlight protean agonism of V2R antagonists.J Biol Chem. 2012 Jan 13;287(3):2099-106. doi: 10.1074/jbc.M111.268797. Epub 2011 Dec 5. J Biol Chem. 2012. PMID: 22144672 Free PMC article.
References
-
- Conklin B R, Bourne H R. Cell. 1993;73:631–641. - PubMed
-
- Gilman A G. Annu Rev Biochem. 1987;56:615–649. - PubMed
-
- Noel J P, Hamm H E, Sigler P B. Nature (London) 1993;366:654–663. - PubMed
-
- Lambright D G, Noel J P, Hamm H E, Sigler P B. Nature (London) 1994;369:621–628. - PubMed
-
- Mixon M B, Lee E, Coleman D E, Berghuis A M, Gilman A G, Sprang S R. Science. 1995;270:954–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

