Proteolytic processing of the Alzheimer disease-associated presenilin-1 generates an in vivo substrate for protein kinase C
- PMID: 9144240
- PMCID: PMC24681
- DOI: 10.1073/pnas.94.10.5349
Proteolytic processing of the Alzheimer disease-associated presenilin-1 generates an in vivo substrate for protein kinase C
Abstract
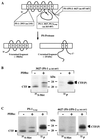
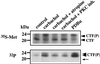
The majority of familial Alzheimer disease mutations are linked to the recently cloned presenilin (PS) genes, which encode two highly homologous proteins (PS-1 and PS-2). It was shown that the full-length PS-2 protein is phosphorylated constitutively within its N-terminal domain by casein kinases, whereas the PS-1 protein is not. Full-length PS proteins undergo endoproteolytic cleavage within their hydrophilic loop domain resulting in the formation of approximately 20-kDa C-terminal fragments (CTF) and approximately 30-kDa N-terminal fragments [Thinakaran, G., et al. (1996) Neuron 17, 181-190]. Here we describe the surprising finding that the CTF of PS-1 is phosphorylated by protein kinase C (PKC). Stimulation of PKC causes a 4- to 5-fold increase of the phosphorylation of the approximately 20-kDa CTF of PS-1 resulting in reduced mobility in SDS gels. PKC-stimulated phosphorylation occurs predominantly on serine residues and can be induced either by direct stimulation of PKC with phorbol-12,13-dibutyrate or by activation of the m1 acetylcholine receptor-signaling pathway with the muscarinic agonist carbachol. However, phosphorylation of full-length PS-1 and PS-2 is not altered upon PKC stimulation. In addition, a mutant form of PS-1 lacking exon 10, which does not undergo endoproteolytic cleavage [Thinakaran, G., et al. (1996) Neuron 17, 181-190] is not phosphorylated by PKC, although it still contains all PKC phosphorylation sites conserved between different species. These results show that PKC phosphorylates the PS-1 CTF. Therefore, endoproteolytic cleavage of full-length PS-1 results in the generation of an in vivo substrate for PKC. The selective phosphorylation of the PS-1 CTF indicates that the physiological and/or pathological properties of the CTF are regulated by PKC activity.
Figures


Similar articles
-
Proteolytic fragments of the Alzheimer's disease associated presenilins-1 and -2 are phosphorylated in vivo by distinct cellular mechanisms.Biochemistry. 1998 Apr 28;37(17):5961-7. doi: 10.1021/bi971763a. Biochemistry. 1998. PMID: 9558331
-
Alzheimer's disease associated presenilin-1 holoprotein and its 18-20 kDa C-terminal fragment are death substrates for proteases of the caspase family.Biochemistry. 1998 Feb 24;37(8):2263-70. doi: 10.1021/bi972106l. Biochemistry. 1998. PMID: 9485372
-
The proteolytic fragments of the Alzheimer's disease-associated presenilin-1 form heterodimers and occur as a 100-150-kDa molecular mass complex.J Biol Chem. 1998 Feb 6;273(6):3205-11. doi: 10.1074/jbc.273.6.3205. J Biol Chem. 1998. PMID: 9452432
-
The Alzheimer's disease-associated presenilins are differentially phosphorylated proteins located predominantly within the endoplasmic reticulum.Mol Med. 1996 Nov;2(6):673-91. Mol Med. 1996. PMID: 8972483 Free PMC article.
-
Proteolytic processing of Alzheimer's disease associated proteins.J Neural Transm Suppl. 1998;53:159-67. doi: 10.1007/978-3-7091-6467-9_14. J Neural Transm Suppl. 1998. PMID: 9700654 Review.
Cited by
-
Effect of caspase cleavage-site phosphorylation on proteolysis.Biochem J. 2003 May 15;372(Pt 1):137-43. doi: 10.1042/BJ20021901. Biochem J. 2003. PMID: 12589706 Free PMC article.
-
Drosophila presenilin is required for neuronal differentiation and affects notch subcellular localization and signaling.J Neurosci. 1999 Oct 1;19(19):8435-42. doi: 10.1523/JNEUROSCI.19-19-08435.1999. J Neurosci. 1999. PMID: 10493744 Free PMC article.
-
Protein Predictive Modeling and Simulation of Mutations of Presenilin-1 Familial Alzheimer's Disease on the Orthosteric Site.Front Mol Biosci. 2021 Jun 2;8:649990. doi: 10.3389/fmolb.2021.649990. eCollection 2021. Front Mol Biosci. 2021. PMID: 34150846 Free PMC article.
-
Evidence for phosphorylation and oligomeric assembly of presenilin 1.Proc Natl Acad Sci U S A. 1997 May 13;94(10):5090-4. doi: 10.1073/pnas.94.10.5090. Proc Natl Acad Sci U S A. 1997. PMID: 9144195 Free PMC article.
-
Trypanosoma cruzi Presenilin-Like Transmembrane Aspartyl Protease: Characterization and Cellular Localization.Biomolecules. 2020 Nov 17;10(11):1564. doi: 10.3390/biom10111564. Biomolecules. 2020. PMID: 33212923 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

