Identification of nitric oxide synthase as a protective locus against tuberculosis
- PMID: 9144222
- PMCID: PMC24663
- DOI: 10.1073/pnas.94.10.5243
Identification of nitric oxide synthase as a protective locus against tuberculosis
Abstract
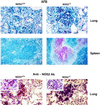
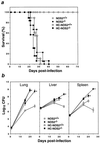
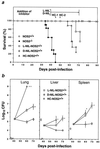
Mutagenesis of the host immune system has helped identify response pathways necessary to combat tuberculosis. Several such pathways may function as activators of a common protective gene: inducible nitric oxide synthase (NOS2). Here we provide direct evidence for this gene controlling primary Mycobacterium tuberculosis infection using mice homozygous for a disrupted NOS2 allele. NOS2(-/-) mice proved highly susceptible, resembling wild-type littermates immunosuppressed by high-dose glucocorticoids, and allowed Mycobacterium tuberculosis to replicate faster in the lungs than reported for other gene-deficient hosts. Susceptibility appeared to be independent of the only known naturally inherited antimicrobial locus, NRAMP1. Progression of chronic tuberculosis in wild-type mice was accelerated by specifically inhibiting NOS2 via administration of N6-(1-iminoethyl)-L-lysine. Together these findings identify NOS2 as a critical host gene for tuberculostasis.
Figures


Similar articles
-
Evidence inconsistent with a role for the Bcg gene (Nramp1) in resistance of mice to infection with virulent Mycobacterium tuberculosis.J Exp Med. 1996 Mar 1;183(3):1045-51. doi: 10.1084/jem.183.3.1045. J Exp Med. 1996. PMID: 8642246 Free PMC article.
-
The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis.Immunology. 1996 Aug;88(4):479-81. doi: 10.1046/j.1365-2567.1996.d01-700.x. Immunology. 1996. PMID: 8881745 Free PMC article.
-
Virulent but not avirulent Mycobacterium tuberculosis can evade the growth inhibitory action of a T helper 1-dependent, nitric oxide Synthase 2-independent defense in mice.J Exp Med. 2002 Oct 7;196(7):991-8. doi: 10.1084/jem.20021186. J Exp Med. 2002. PMID: 12370260 Free PMC article.
-
Significance of the antimicrobial resistance gene, Nramp1, in resistance to virulent Mycobacterium tuberculosis infection.Res Immunol. 1996 Oct-Dec;147(8-9):493-9. doi: 10.1016/s0923-2494(97)85213-3. Res Immunol. 1996. PMID: 9127879 Review. No abstract available.
-
NADPH oxidase, Nramp1 and nitric oxide synthase 2 in the host antimicrobial response.Rev Immunogenet. 2000;2(3):387-415. Rev Immunogenet. 2000. PMID: 11256747 Review.
Cited by
-
Inducible antibacterial responses in macrophages.Nat Rev Immunol. 2025 Feb;25(2):92-107. doi: 10.1038/s41577-024-01080-y. Epub 2024 Sep 18. Nat Rev Immunol. 2025. PMID: 39294278 Review.
-
Toll-like receptor 2-dependent extracellular signal-regulated kinase signaling in Mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits Th1 polarization of responding T cells.Infect Immun. 2015 Jun;83(6):2242-54. doi: 10.1128/IAI.00135-15. Epub 2015 Mar 16. Infect Immun. 2015. PMID: 25776754 Free PMC article.
-
Resistance and Susceptibility Immune Factors at Play during Mycobacterium tuberculosis Infection of Macrophages.Pathogens. 2022 Oct 6;11(10):1153. doi: 10.3390/pathogens11101153. Pathogens. 2022. PMID: 36297211 Free PMC article. Review.
-
Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-guerin and Mycobacterium tuberculosis infections.Am J Pathol. 2005 Apr;166(4):1109-20. doi: 10.1016/S0002-9440(10)62331-0. Am J Pathol. 2005. PMID: 15793291 Free PMC article.
-
Toll-like receptors: molecular mechanisms of the mammalian immune response.Immunology. 2000 Sep;101(1):1-10. doi: 10.1046/j.1365-2567.2000.00093.x. Immunology. 2000. PMID: 11012747 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

