Involvement of caspase-dependent activation of cytosolic phospholipase A2 in tumor necrosis factor-induced apoptosis
- PMID: 9144192
- PMCID: PMC24633
- DOI: 10.1073/pnas.94.10.5073
Involvement of caspase-dependent activation of cytosolic phospholipase A2 in tumor necrosis factor-induced apoptosis
Abstract
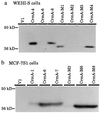
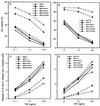
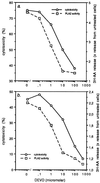
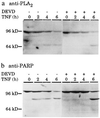
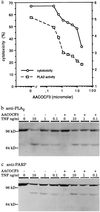
Tumor necrosis factor (TNF)-induced apoptosis is mediated by caspases, which are cysteine proteases related to interleukin 1beta-converting enzyme. We report here that TNF-induced activation of caspases results in the cleavage and activation of cytosolic phospholipase A2 (cPLA2) and that activated cPLA2 contributes to apoptosis. Inhibition of caspases by expression of a cowpox virus-derived inhibitor, CrmA, or by a specific tetrapeptide inhibitor of CPP32/caspase-3, acetyl-Asp-Glu-Val-Asp-aldehyde (Ac-DEVD-CHO), inhibited TNF-induced activation of cPLA2 and apoptosis. TNF-induced activation of cPLA2 was accompanied by a cleavage of the 100-kDa cPLA2 to a 70-kDa proteolytic fragment. This cleavage was inhibited by Ac-DEVD-CHO in a similar manner as that of poly(ADP)ribose polymerase, a known substrate of CPP32/caspase-3. Interestingly, specific inhibition of cPLA2 enzyme activity by arachidonyl trifluoromethylketone (AACOCF3) partially inhibited TNF-induced apoptosis without inhibition of caspase activity. Thus, our results suggest a novel caspase-dependent activation pathway for cPLA2 during apoptosis and identify cPLA2 as a mediator of TNF-induced cell death acting downstream of caspases.
Figures
Similar articles
-
Specific cleavage of alpha-fodrin during Fas- and tumor necrosis factor-induced apoptosis is mediated by an interleukin-1beta-converting enzyme/Ced-3 protease distinct from the poly(ADP-ribose) polymerase protease.J Biol Chem. 1996 Dec 6;271(49):31277-82. doi: 10.1074/jbc.271.49.31277. J Biol Chem. 1996. PMID: 8940132
-
Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation.J Biol Chem. 1998 May 29;273(22):13870-7. doi: 10.1074/jbc.273.22.13870. J Biol Chem. 1998. PMID: 9593733
-
Cleavage of human cytosolic phospholipase A2 by caspase-1 (ICE) and caspase-8 (FLICE).Biochem Biophys Res Commun. 1998 Dec 9;253(1):92-8. doi: 10.1006/bbrc.1998.9754. Biochem Biophys Res Commun. 1998. PMID: 9875225
-
Recent insights into the structure, function and biology of cPLA2.Agents Actions Suppl. 1995;46:65-76. doi: 10.1007/978-3-0348-7276-8_7. Agents Actions Suppl. 1995. PMID: 7610992 Review.
-
Role of caspase-1 subfamily in cytotoxic cytokine-induced oligodendrocyte cell death.J Neural Transm Suppl. 2000;(58):135-42. doi: 10.1007/978-3-7091-6284-2_11. J Neural Transm Suppl. 2000. PMID: 11128603 Review.
Cited by
-
Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine.J Biol Chem. 2006 Aug 4;281(31):22275-22288. doi: 10.1074/jbc.M604330200. Epub 2006 May 25. J Biol Chem. 2006. PMID: 16728389 Free PMC article.
-
Annexin-II, DNA, and histones serve as factor H ligands on the surface of apoptotic cells.J Biol Chem. 2010 Feb 5;285(6):3766-3776. doi: 10.1074/jbc.M109.045427. Epub 2009 Dec 1. J Biol Chem. 2010. PMID: 19951950 Free PMC article.
-
PLIP, a novel splice variant of Tip60, interacts with group IV cytosolic phospholipase A(2), induces apoptosis, and potentiates prostaglandin production.Mol Cell Biol. 2001 Jul;21(14):4470-81. doi: 10.1128/MCB.21.14.4470-4481.2001. Mol Cell Biol. 2001. PMID: 11416127 Free PMC article.
-
Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins.J Virol. 2001 Oct;75(19):8875-87. doi: 10.1128/JVI.75.19.8875-8887.2001. J Virol. 2001. PMID: 11533151 Free PMC article.
-
Regulation of membrane release in apoptosis.Biochem J. 1998 Sep 1;334 ( Pt 2)(Pt 2):479-85. doi: 10.1042/bj3340479. Biochem J. 1998. PMID: 9716508 Free PMC article.
References
-
- White E. Genes Dev. 1996;10:1–15. - PubMed
-
- Yuan J-Y, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. - PubMed
-
- Tartaglia L A, Ayers T M, Wong G H W, Goeddel D V. Cell. 1993;74:845–853. - PubMed
-
- Itoh N, Nagata S. J Biol Chem. 1993;268:10932–10937. - PubMed
-
- Hsu H, Xiong J, Goeddel D V. Cell. 1995;81:495–504. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

