Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation
- PMID: 9108048
- PMCID: PMC20511
- DOI: 10.1073/pnas.94.8.3742
Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation
Abstract
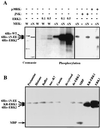
Mitogen-activated protein kinase kinase (MEK) is a dual-specificity protein kinase that is located primarily in the cellular cytosol, both prior to and upon mitogenic stimulation. The existence of a nuclear export signal in the N-terminal domain of MEK [Fukuda, M., Gotoh, I., Gotoh, Y. & Nishida, E. (1996) J. Biol. Chem. 271, 20024-20028] suggests that there are circumstances under which MEK enters the nucleus and must be exported. Using mutants of MEK, we show that the deletion of the nuclear export signal sequence from constitutively active MEK caused constitutive localization of MEK in the nucleus of COS7 and HEK-293T cells. However, when the same region was deleted from a catalytically inactive MEK, cytoplasmic localization was observed in resting cells, which turned nuclear upon stimulation. Confocal microscopy of COS7 cells expressing the above mutants showed localization of the active MEK in the nuclear envelope and also in the cell periphery. The differences in cellular localization between the wild-type and mutant MEKs are not due to severe changes in specificity because the recombinant, constitutively active MEK that lacked its N-terminal region exhibited the same substrate specificity as the wild-type MEK, both in vitro and in intact cells. Taken together, our results indicate that upon mitogenic stimulation, MEK, like extracellular signal responsive kinase and p90(RSK), is massively translocated to the nucleus. Rapid export from the nucleus, which is mediated by the nuclear export signal, is probably the cause for the cytoplasmic distribution observed with wild-type MEK.
Figures

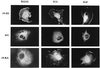
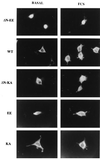
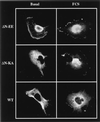
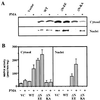

Similar articles
-
Cytoplasmic localization of the mitogen-activated protein kinase activator MEK.J Biol Chem. 1994 Aug 5;269(31):19947-52. J Biol Chem. 1994. PMID: 8051079
-
Non-regulated and stimulated mechanisms cooperate in the nuclear accumulation of MEK1.Oncogene. 2001 Nov 15;20(52):7588-96. doi: 10.1038/sj.onc.1204963. Oncogene. 2001. PMID: 11753637
-
Insulin regulation of mitogen-activated protein kinase kinase (MEK), mitogen-activated protein kinase and casein kinase in the cell nucleus: a possible role in the regulation of gene expression.Biochem J. 1997 May 1;323 ( Pt 3)(Pt 3):621-7. doi: 10.1042/bj3230621. Biochem J. 1997. PMID: 9169593 Free PMC article.
-
Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2.Curr Biol. 1998 Sep 24;8(19):1049-57. doi: 10.1016/s0960-9822(98)70442-7. Curr Biol. 1998. PMID: 9768359 Review.
-
MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma.Cell Cycle. 2007 Jul 1;6(13):1539-48. doi: 10.4161/cc.6.13.4453. Epub 2007 May 18. Cell Cycle. 2007. PMID: 17611413 Review.
Cited by
-
Nuclear translocation of MEK1 triggers a complex T cell response through the corepressor silencing mediator of retinoid and thyroid hormone receptor.J Immunol. 2013 Jan 1;190(1):159-67. doi: 10.4049/jimmunol.1201657. Epub 2012 Dec 5. J Immunol. 2013. PMID: 23225884 Free PMC article.
-
Extracellular-Regulated Kinases: Signaling From Ras to ERK Substrates to Control Biological Outcomes.Adv Cancer Res. 2018;138:99-142. doi: 10.1016/bs.acr.2018.02.004. Epub 2018 Mar 2. Adv Cancer Res. 2018. PMID: 29551131 Free PMC article. Review.
-
The MKK7 gene encodes a group of c-Jun NH2-terminal kinase kinases.Mol Cell Biol. 1999 Feb;19(2):1569-81. doi: 10.1128/MCB.19.2.1569. Mol Cell Biol. 1999. PMID: 9891090 Free PMC article.
-
Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry.EMBO J. 1999 Feb 1;18(3):664-74. doi: 10.1093/emboj/18.3.664. EMBO J. 1999. PMID: 9927426 Free PMC article.
-
The ERK cascade: a prototype of MAPK signaling.Mol Biotechnol. 2005 Oct;31(2):151-74. doi: 10.1385/MB:31:2:151. Mol Biotechnol. 2005. PMID: 16170216 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

