Telomeres cluster de novo before the initiation of synapsis: a three-dimensional spatial analysis of telomere positions before and during meiotic prophase
- PMID: 9105032
- PMCID: PMC2139864
- DOI: 10.1083/jcb.137.1.5
Telomeres cluster de novo before the initiation of synapsis: a three-dimensional spatial analysis of telomere positions before and during meiotic prophase
Abstract
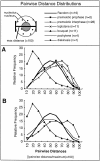
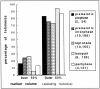
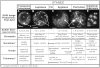
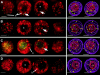
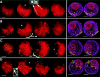
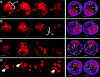
We have analyzed the progressive changes in the spatial distribution of telomeres during meiosis using three-dimensional, high resolution fluorescence microscopy. Fixed meiotic cells of maize (Zea mays L.) were subjected to in situ hybridization under conditions that preserved chromosome structure, allowing identification of stage-dependent changes in telomere arrangements. We found that nuclei at the last somatic prophase before meiosis exhibit a nonrandom, polarized chromosome organization resulting in a loose grouping of telomeres. Quantitative measurements on the spatial arrangements of telomeres revealed that, as cells passed through premeiotic interphase and into leptotene, there was an increase in the frequency of large telomere-to-telomere distances and a decrease in the bias toward peripheral localization of telomeres. By leptotene, there was no obvious evidence of telomere grouping, and the large, singular nucleolus was internally located, nearly concentric with the nucleus. At the end of leptotene, telomeres clustered de novo at the nuclear periphery, coincident with a displacement of the nucleolus to one side. The telomere cluster persisted throughout zygotene and into early pachytene. The nucleolus was adjacent to the cluster at zygotene. At the pachytene stage, telomeres rearranged again by dispersing throughout the nuclear periphery. The stage-dependent changes in telomere arrangements are suggestive of specific, active telomere-associated motility processes with meiotic functions. Thus, the formation of the cluster itself is an early event in the nuclear reorganizations associated with meiosis and may reflect a control point in the initiation of synapsis or crossing over.
Figures




Similar articles
-
Nucleolus-associated telomere clustering and pairing precede meiotic chromosome synapsis in Arabidopsis thaliana.J Cell Sci. 2001 Dec;114(Pt 23):4207-17. doi: 10.1242/jcs.114.23.4207. J Cell Sci. 2001. PMID: 11739653
-
Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing.J Cell Biol. 1996 Sep;134(5):1109-25. doi: 10.1083/jcb.134.5.1109. J Cell Biol. 1996. PMID: 8794855 Free PMC article.
-
The desynaptic (dy) and desynaptic1 (dsy1) mutations in maize (Zea mays L) cause distinct telomere-misplacement phenotypes during meiotic prophase.J Exp Bot. 2003 Jan;54(380):39-46. doi: 10.1093/jxb/erg032. J Exp Bot. 2003. PMID: 12456753
-
Telomere distribution and dynamics in somatic and meiotic nuclei of Arabidopsis thaliana.Cytogenet Genome Res. 2009;124(3-4):193-201. doi: 10.1159/000218125. Epub 2009 Jun 25. Cytogenet Genome Res. 2009. PMID: 19556773 Review.
-
The molecular control of meiotic chromosomal behavior: events in early meiotic prophase in Drosophila oocytes.Annu Rev Physiol. 2012;74:425-51. doi: 10.1146/annurev-physiol-020911-153342. Annu Rev Physiol. 2012. PMID: 22335798 Review.
Cited by
-
Molecular cytogenetic analysis of telomere rearrangements.Curr Protoc Hum Genet. 2015 Jan 20;84:8.11.1-8.11.15. doi: 10.1002/0471142905.hg0811s84. Curr Protoc Hum Genet. 2015. PMID: 25599669 Free PMC article.
-
Dynamics of rye chromosome 1R regions with high or low crossover frequency in homology search and synapsis development.PLoS One. 2012;7(4):e36385. doi: 10.1371/journal.pone.0036385. Epub 2012 Apr 30. PLoS One. 2012. PMID: 22558456 Free PMC article.
-
3D Molecular Cytology of Hop (Humulus lupulus) Meiotic Chromosomes Reveals Non-disomic Pairing and Segregation, Aneuploidy, and Genomic Structural Variation.Front Plant Sci. 2018 Nov 1;9:1501. doi: 10.3389/fpls.2018.01501. eCollection 2018. Front Plant Sci. 2018. PMID: 30443259 Free PMC article.
-
Bouquet Formation Failure in Meiosis of F1 Wheat-Rye Hybrids with Mitotic-Like Division.Plants (Basel). 2022 Jun 15;11(12):1582. doi: 10.3390/plants11121582. Plants (Basel). 2022. PMID: 35736732 Free PMC article.
-
A dynamic meiotic SUN belt includes the zygotene-stage telomere bouquet and is disrupted in chromosome segregation mutants of maize (Zea mays L.).Front Plant Sci. 2014 Jul 11;5:314. doi: 10.3389/fpls.2014.00314. eCollection 2014. Front Plant Sci. 2014. PMID: 25071797 Free PMC article.
References
-
- Bennett MD, Smith JB, Heslop-Harrison JS. Nuclear DNA amounts in angiosperms. Proc R Soc Lond Ser B. 1983;216:179–199. - PubMed
-
- Burns JA. Preleptotene chromosome contraction in Nicotianaspecies. J Hered. 1972;63:175–178.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

