RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis
- PMID: 9104823
- PMCID: PMC2196251
- DOI: 10.1084/jem.185.7.1371
RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis
Abstract
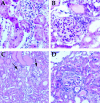
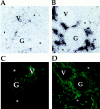
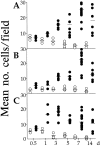
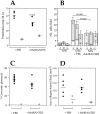
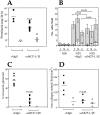
The involvement of chemokines in inflammation is well established, but their functional role in disease progression, and particularly in the development of fibrosis, is not yet understood. To investigate the functional role that the chemokines monocyte chemoattractant protein-1 (MCP-1) and RANTES play in inflammation and the progression to fibrosis during crescentic nephritis we have developed and characterized a murine model for this syndrome. Significant increases in T-lymphocytes and macrophages were observed within glomeruli and interstitium, paralleled by an induction of mRNA expression of MCP-1 and RANTES, early after disease initiation. Blocking the function of MCP-1 or RANTES resulted in significant decreases in proteinuria as well as in numbers of infiltrating leukocytes, indicating that both MCP-1 and RANTES (regulated upon activation in normal T cells expressed and secreted) play an important role in the inflammatory phase of crescentic nephritis. In addition, neutralization of MCP-1 resulted in a dramatic decrease in both glomerular crescent formation and deposition of type I collagen. These results highlight a novel role for MCP-1 in crescent formation and development of interstitial fibrosis, and indicate that in addition to recruiting inflammatory cells this chemokine is critically involved in irreversible tissue damage.
Figures
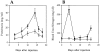
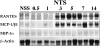
Similar articles
-
Role of MCP-1 and RANTES in inflammation and progression to fibrosis during murine crescentic nephritis.J Leukoc Biol. 1997 Nov;62(5):676-80. doi: 10.1002/jlb.62.5.676. J Leukoc Biol. 1997. PMID: 9365123
-
Expression of the chemokines MCP-1/CCL2 and RANTES/CCL5 is differentially regulated by infiltrating inflammatory cells.Kidney Int. 2002 Oct;62(4):1264-76. doi: 10.1111/j.1523-1755.2002.kid572.x. Kidney Int. 2002. PMID: 12234296
-
Glomerular expression of C-C chemokines in different types of human crescentic glomerulonephritis.Nephrol Dial Transplant. 2003 Aug;18(8):1526-34. doi: 10.1093/ndt/gfg172. Nephrol Dial Transplant. 2003. PMID: 12897090
-
MCP-1 and RANTES are mediators of acute and chronic inflammation.Allergy Asthma Proc. 2001 May-Jun;22(3):133-7. doi: 10.2500/108854101778148737. Allergy Asthma Proc. 2001. PMID: 11424873 Review.
-
Role of MCP-1 as an inflammatory biomarker in nephropathy.Front Immunol. 2024 Jan 4;14:1303076. doi: 10.3389/fimmu.2023.1303076. eCollection 2023. Front Immunol. 2024. PMID: 38239353 Free PMC article. Review.
Cited by
-
Infectious disease, the innate immune response, and fibrosis.J Clin Invest. 2007 Mar;117(3):530-8. doi: 10.1172/JCI30595. J Clin Invest. 2007. PMID: 17332880 Free PMC article. Review.
-
Spatiotemporal expression of chemokines and chemokine receptors in experimental anti-myeloperoxidase antibody-mediated glomerulonephritis.Clin Exp Immunol. 2009 Oct;158(1):143-53. doi: 10.1111/j.1365-2249.2009.03993.x. Clin Exp Immunol. 2009. PMID: 19737241 Free PMC article.
-
Cellular and molecular mechanisms of fibrosis.J Pathol. 2008 Jan;214(2):199-210. doi: 10.1002/path.2277. J Pathol. 2008. PMID: 18161745 Free PMC article. Review.
-
The motor protein Myo1c regulates transforming growth factor-β-signaling and fibrosis in podocytes.Kidney Int. 2019 Jul;96(1):139-158. doi: 10.1016/j.kint.2019.02.014. Epub 2019 Mar 4. Kidney Int. 2019. PMID: 31097328 Free PMC article.
-
Regionally Distinct Immune and Metabolic Transcriptional Responses in the Bovine Small Intestine and Draining Lymph Nodes During a Subclinical Mycobacterium avium subsp. paratuberculosis Infection.Front Immunol. 2021 Dec 15;12:760931. doi: 10.3389/fimmu.2021.760931. eCollection 2021. Front Immunol. 2021. PMID: 34975852 Free PMC article.
References
-
- Risdon RA, Sloper JC, DeWardener HE. Relationship between renal function and histological changes found in renal biopsy specimens from patients with persistent glomerulonephritis. Lancet. 1968;2:363–366. - PubMed
-
- Bohle A, Wehrmann M, Bogenschultz O, Vogel W, Schmitt H, Muller CA, Muller GA. The long term prognosis of the primary glomerulonephritides. A morphologic and clinical analysis of 1747 cases. Pathol Res Pract. 1992;188:908–924. - PubMed
-
- Schreiner GF. The macrophage in glomerular injury. Seminar Nephrol. 1991;11:268–275. - PubMed
-
- Sado Y, Naito I, Akita M, Okigaki T. Strain specific responses of inbred rats on the severity of experimental autoimune glomerulonephritis. J Clin Lab Immunol. 1986;19:193–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

