cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein
- PMID: 9096337
- PMCID: PMC20313
- DOI: 10.1073/pnas.94.7.3010
cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein
Erratum in
- Proc Natl Acad Sci U S A 1997 Sep 2;94(18):10006
Abstract
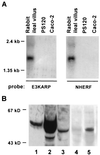
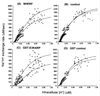
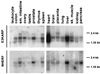
NHE3 is the Na+/H+ exchanger located on the intestinal and renal brush border membrane, where it functions in transepithelial Na+ absorption. The brush border Na+ absorptive process is acutely inhibited by activation of cAMP-dependent protein kinase, but the molecular mechanism of this inhibitory effect is poorly understood. We have identified two regulatory proteins, E3KARP and NHERF, that interact with NHE3 to enable cAMP to inhibit NHE3. The two regulatory proteins are structurally related, sharing approximately 50% identity in amino acid sequences. It has been previously shown that when NHE3 is transfected into PS120 fibroblasts or Caco-2 cells, cAMP failed to inhibit NHE3 activity. Northern blot analysis showed that both PS120 and Caco-2 cells lacked the expression of both E3KARP and NHERF. In contrast, other cell lines in which cAMP inhibits NHE3, including OK, CHO, and LLC-PK1 cells, expressed NHERF-related regulatory proteins. To determine their functions in cAMP-dependent inhibition of NHE3, E3KARP and NHERF were transfected into PS120/NHE3 fibroblasts. Transfection in PS120/NHE3 fibroblasts with either NHERF or E3KARP reconstituted cAMP-induced inhibition of NHE3, resulting in 25-30% inhibition in these cells.
Figures

Similar articles
-
NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin.J Biol Chem. 1998 Oct 2;273(40):25856-63. doi: 10.1074/jbc.273.40.25856. J Biol Chem. 1998. PMID: 9748260
-
cAMP-induced phosphorylation and inhibition of Na(+)/H(+) exchanger 3 (NHE3) are dependent on the presence but not the phosphorylation of NHE regulatory factor.J Biol Chem. 1999 Aug 27;274(35):24753-8. doi: 10.1074/jbc.274.35.24753. J Biol Chem. 1999. PMID: 10455146
-
The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3.J Biol Chem. 1998 Nov 6;273(45):29972-8. doi: 10.1074/jbc.273.45.29972. J Biol Chem. 1998. PMID: 9792717
-
Short-term regulation of NHE3 by EGF and protein kinase C but not protein kinase A involves vesicle trafficking in epithelial cells and fibroblasts.Ann N Y Acad Sci. 2000;915:30-42. doi: 10.1111/j.1749-6632.2000.tb05221.x. Ann N Y Acad Sci. 2000. PMID: 11193592 Review.
-
Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA.Am J Physiol Renal Physiol. 2000 Sep;279(3):F393-9. doi: 10.1152/ajprenal.2000.279.3.F393. Am J Physiol Renal Physiol. 2000. PMID: 10966919 Review.
Cited by
-
Ubiquitin-specific peptidase 7 (USP7) and USP10 mediate deubiquitination of human NHE3 regulating its expression and activity.FASEB J. 2020 Dec;34(12):16476-16488. doi: 10.1096/fj.202001875R. Epub 2020 Oct 23. FASEB J. 2020. PMID: 33095475 Free PMC article.
-
Regulation of glomerulotubular balance. I. Impact of dopamine on flow-dependent transport.Am J Physiol Renal Physiol. 2012 Aug 1;303(3):F386-95. doi: 10.1152/ajprenal.00531.2011. Epub 2012 May 2. Am J Physiol Renal Physiol. 2012. PMID: 22552936 Free PMC article.
-
Protein/protein interactions (PDZ) in proximal tubules.J Membr Biol. 2005 Feb;203(3):111-8. doi: 10.1007/s00232-005-0738-7. J Membr Biol. 2005. PMID: 15986090 Review. No abstract available.
-
Intracellular pH regulation by Na(+)/H(+) exchange requires phosphatidylinositol 4,5-bisphosphate.J Cell Biol. 2000 Jul 10;150(1):213-24. doi: 10.1083/jcb.150.1.213. J Cell Biol. 2000. PMID: 10893269 Free PMC article.
-
Designing vaccines to neutralize effective toxin delivery by enterotoxigenic Escherichia coli.Toxins (Basel). 2014 Jun 10;6(6):1799-812. doi: 10.3390/toxins6061799. Toxins (Basel). 2014. PMID: 24918359 Free PMC article. Review.
References
-
- Donowitz M, Welsh M J. Annu Rev Physiol. 1986;48:135–150. - PubMed
-
- Weinman E J, Shnolikar S. Annu Rev Physiol. 1993;55:289–304. - PubMed
-
- Yun C H C, Tse C-M, Nath S K, Levine S A, Brant S R, Donowitz M. Am J Physiol. 1995;269:G1–G11. - PubMed
-
- Aronson P S, Igarashi P. Curr Top Membr Transp. 1986;26:57–75.
-
- Levine S A, Montrose M H, Tse C M, Donowitz M. J Biol Chem. 1993;268:25527–25535. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

