Nonuniform distribution of Ca2+ channel subtypes on presynaptic terminals of excitatory synapses in hippocampal cultures
- PMID: 9092595
- PMCID: PMC6573101
- DOI: 10.1523/JNEUROSCI.17-08-02738.1997
Nonuniform distribution of Ca2+ channel subtypes on presynaptic terminals of excitatory synapses in hippocampal cultures
Abstract
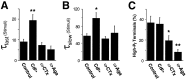
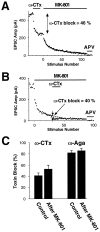
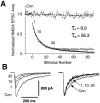
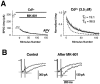
Several subtypes of Ca2+ channel support the release of glutamate at excitatory synapses. We investigated the pattern of colocalization of these subtypes on presynaptic terminals in hippocampal cultures. N-type (conotoxin GVIA-sensitive) or P/Q-type (agatoxin IVA-sensitive) Ca2+ channels were blocked selectively, and the reduction in transmitter release probability (Pr) was measured with MK-801. The antagonists completely blocked release at some terminals, reduced Pr at others, and failed to affect the remainder. In contrast, nonselective reduction of presynaptic Ca2+ influx by adding Cd2+ or lowering external Ca2+ reduced Pr uniformly at all terminals. We conclude from these results that the mixture of N-type and P/Q-type channels varies markedly between terminals on the same afferent. The distribution of Ca2+ channel subtypes was the same for high and low Pr terminals. Given that Ca2+ channel subtypes are affected differentially by neuromodulators, these findings lead to the possibility of terminal-specific modulation of synaptic function.
Figures

Similar articles
-
The use of invertebrate peptide toxins to establish Ca2+ channel identity of CA3-CA1 neurotransmission in rat hippocampal slices.Eur J Pharmacol. 1996 Jun 13;306(1-3):41-50. doi: 10.1016/0014-2999(96)00195-1. Eur J Pharmacol. 1996. PMID: 8813613
-
Developmental changes in presynaptic calcium channels coupled to glutamate release in cultured rat hippocampal neurons.J Neurosci. 1995 Jun;15(6):4612-7. doi: 10.1523/JNEUROSCI.15-06-04612.1995. J Neurosci. 1995. PMID: 7790927 Free PMC article.
-
N- and P/Q-type Ca2+ channels mediate transmitter release with a similar cooperativity at rat hippocampal autapses.J Neurosci. 1998 Apr 15;18(8):2849-55. doi: 10.1523/JNEUROSCI.18-08-02849.1998. J Neurosci. 1998. PMID: 9526002 Free PMC article.
-
Presynaptic excitability.Int Rev Neurobiol. 1995;38:201-51. doi: 10.1016/s0074-7742(08)60527-9. Int Rev Neurobiol. 1995. PMID: 8537201 Review.
-
Synaptogenesis: new roles for an old player.Curr Biol. 2009 Dec 29;19(24):R1114-5. doi: 10.1016/j.cub.2009.10.064. Curr Biol. 2009. PMID: 20064419 Free PMC article. Review.
Cited by
-
L-type Ca2+ channels mediate regulation of glutamate release by subthreshold potential changes.Proc Natl Acad Sci U S A. 2023 Mar 21;120(12):e2220649120. doi: 10.1073/pnas.2220649120. Epub 2023 Mar 15. Proc Natl Acad Sci U S A. 2023. PMID: 36920925 Free PMC article.
-
Presynaptic L-Type Ca2+ Channels Increase Glutamate Release Probability and Excitatory Strength in the Hippocampus during Chronic Neuroinflammation.J Neurosci. 2020 Sep 2;40(36):6825-6841. doi: 10.1523/JNEUROSCI.2981-19.2020. Epub 2020 Aug 3. J Neurosci. 2020. PMID: 32747440 Free PMC article.
-
Brain-derived neurotrophic factor enhances GABA release probability and nonuniform distribution of N- and P/Q-type channels on release sites of hippocampal inhibitory synapses.J Neurosci. 2005 Mar 30;25(13):3358-68. doi: 10.1523/JNEUROSCI.4227-04.2005. J Neurosci. 2005. PMID: 15800191 Free PMC article.
-
Regional differences in the effects of isoflurane on neurotransmitter release.Neuropharmacology. 2011 Sep;61(4):699-706. doi: 10.1016/j.neuropharm.2011.05.013. Epub 2011 May 30. Neuropharmacology. 2011. PMID: 21651920 Free PMC article.
-
The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore.J Neurosci. 2009 Mar 25;29(12):3781-91. doi: 10.1523/JNEUROSCI.5512-08.2009. J Neurosci. 2009. PMID: 19321774 Free PMC article.
References
-
- Castillo PE, Weisskopf MG, Nicoll RA. The role of Ca2+ channels in hippocampal mossy fiber synaptic transmission and long-term potentiation. Neuron. 1994;12:261–269. - PubMed
-
- Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. - PubMed
-
- Fujita Y, Mynlieff M, Dirksen RT, Kim MS, Niidome T, Nakai J, Friedrich T, Iwabe N, Miyata T, Furuichi T, Furutama D, Mikoshiba K, Mori Y, Beam KG. Primary structure and functional expression of the omega-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10:585–598. - PubMed
-
- Glaum SR, Miller RJ. Presynaptic metabotropic glutamate receptors modulate omega-conotoxin-GVIA-insensitive calcium channels in the rat medulla. Neuropharmacology. 1995;34:953–964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
