Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells
- PMID: 9087435
- PMCID: PMC2132506
- DOI: 10.1083/jcb.136.6.1169
Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner centromere protein positioning in mitotic heterokaryons and mid-anaphase cells
Abstract
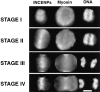
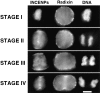
After the separation of sister chromatids in anaphase, it is essential that the cell position a cleavage furrow so that it partitions the chromatids into two daughter cells of roughly equal size. The mechanism by which cells position this cleavage furrow remains unknown, although the best current model is that furrows always assemble midway between asters. We used micromanipulation of human cultured cells to produce mitotic heterokaryons with two spindles fused in a V conformation. The majority (15/19) of these cells cleaved along a single plane that transected the two arms of the V at the position where the metaphase plate had been, a result at odds with current views of furrow positioning. However, four cells did form an additional ectopic furrow between the spindle poles at the open end of the V, consistent with the established view. To begin to address the mechanism of furrow assembly, we have begun a detailed study of the properties of the chromosome passenger inner centromere protein (INCENP) in anaphase and telophase cells. We found that INCENP is a very early component of the cleavage furrow, accumulating at the equatorial cortex before any noticeable cortical shape change and before any local accumulation of myosin heavy chain. In mitotic heterokaryons, INCENP was detected in association with spindle midzone microtubules beneath sites of furrowing and was not detected when furrows were absent. A functional role for INCENP in cytokinesis was suggested in experiments where a nearly full-length INCENP was tethered to the centromere. Many cells expressing the chimeric INCENP failed to complete cytokinesis and entered the next cell cycle with daughter cells connected by a large intercellular bridge with a prominent midbody. Together, these results suggest that INCENP has a role in either the assembly or function of the cleavage furrow.
Figures


Similar articles
-
Two mechanisms coordinate the recruitment of the chromosomal passenger complex to the plane of cell division.Mol Biol Cell. 2017 Dec 1;28(25):3634-3646. doi: 10.1091/mbc.E17-06-0399. Epub 2017 Sep 27. Mol Biol Cell. 2017. PMID: 28954866 Free PMC article.
-
Colocalization of TD-60 and INCENP throughout G2 and mitosis: evidence for their possible interaction in signalling cytokinesis.Chromosoma. 1998 Dec;107(6-7):461-70. doi: 10.1007/s004120050330. Chromosoma. 1998. PMID: 9914378
-
INCENP binds directly to tubulin and requires dynamic microtubules to target to the cleavage furrow.Exp Cell Res. 2001 Jan 15;262(2):122-7. doi: 10.1006/excr.2000.5088. Exp Cell Res. 2001. PMID: 11139336
-
Role of chromosomal passenger complex in chromosome segregation and cytokinesis.Cell Struct Funct. 2001 Dec;26(6):653-7. doi: 10.1247/csf.26.653. Cell Struct Funct. 2001. PMID: 11942622 Review.
-
Establishment of the mechanism of cytokinesis in animal cells.Int Rev Cytol. 1986;105:245-81. doi: 10.1016/s0074-7696(08)61065-7. Int Rev Cytol. 1986. PMID: 3539854 Review.
Cited by
-
Incomplete sister chromatid separation of long chromosome arms.Chromosoma. 2006 Dec;115(6):481-90. doi: 10.1007/s00412-006-0077-1. Epub 2006 Oct 5. Chromosoma. 2006. PMID: 17021850
-
Essential roles of KIF4 and its binding partner PRC1 in organized central spindle midzone formation.EMBO J. 2004 Aug 18;23(16):3237-48. doi: 10.1038/sj.emboj.7600347. Epub 2004 Aug 5. EMBO J. 2004. PMID: 15297875 Free PMC article.
-
MCAK facilitates chromosome movement by promoting kinetochore microtubule turnover.J Cell Biol. 2007 Dec 3;179(5):869-79. doi: 10.1083/jcb.200707120. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039936 Free PMC article.
-
Microtubules are the only structural constituent of the spindle apparatus required for induction of cell cleavage.J Cell Biol. 2003 Aug 4;162(3):383-90. doi: 10.1083/jcb.200301073. J Cell Biol. 2003. PMID: 12900392 Free PMC article.
-
Loss of Ewing sarcoma EWS allele promotes tumorigenesis by inducing chromosomal instability in zebrafish.Sci Rep. 2016 Aug 25;6:32297. doi: 10.1038/srep32297. Sci Rep. 2016. PMID: 27557633 Free PMC article.
References
-
- Andreassen PR, Palmer DK, Wener MH, Margolis RL. Telophase disk: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99:523–534. - PubMed
-
- Aubin JE, Osborn M, Weber K. Inhibition of cytokinesis and altered contractile ring morphology induced by cytochalasins in synchronized PtK2 cells. Exp Cell Res. 1981;136:63–79. - PubMed
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1991. Current Protocols in Molecular Biology. John Wiley & Sons, New York.
-
- Bray D, White JG. Cortical flow in animal cells. Science (Wash DC) 1988;239:883–888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

